EGFR (epidermal growth factor receptor) inhibitor for treating cancer and synthesis method of EGFR inhibitor
A technology of epidermal growth factor and receptor inhibitors, applied in the field of drug synthesis, can solve problems such as unclear roles, and achieve the effect of easy purchase of raw materials, simple operation, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
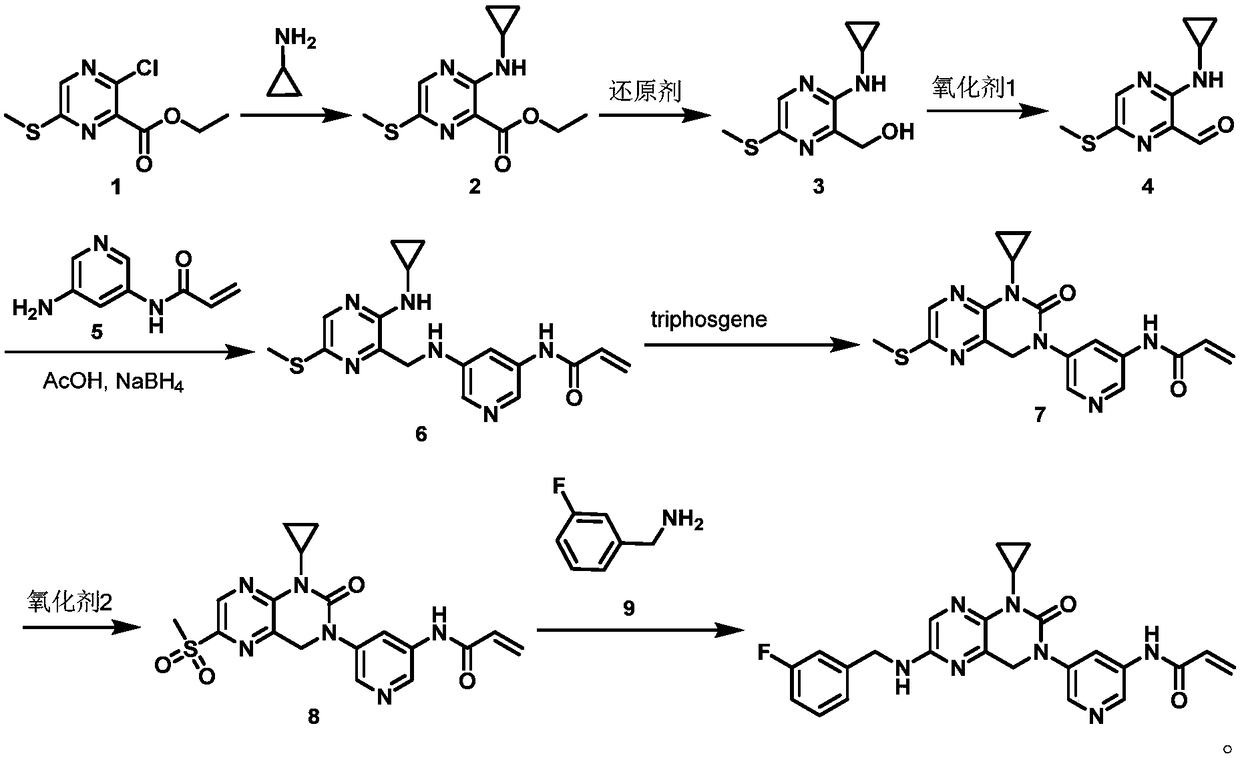
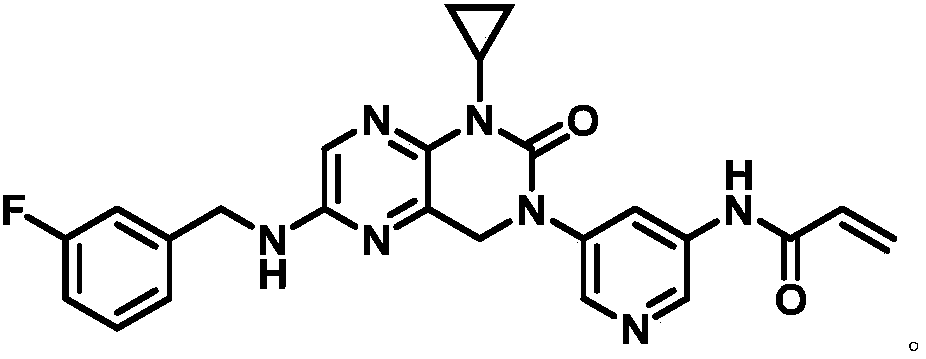
[0040] The synthetic conditions of 3-cyclopropylamino-6-methylthio-2-pyrazinecarboxylic acid ethyl ester 2 are:
[0041] Mix ethyl 3-chloro-6-methylthio-2-pyrazinecarboxylate 1 (35.8g), cyclopropylamine (13.18g), potassium carbonate (44.65g) and dimethylformamide (358mL) and heat to Stir at 60-100°C for 8-14 hours; cool down to 15-25°C, add ice water (700mL) to the system, stir for 2-5 hours, and filter to obtain 3-cyclopropylamino-6-methylthio - Ethyl 2-pyrazinecarboxylate 2 (34.9 g, 90%).
[0042] 1 H NMR (CDCl 3 ,400MHz): δ(ppm)=8.42(s,1H),7.56(brs,1H),4.23(q,2H),2.59(s,3H),2.29(m,1H),1.29(t,3H) ,0.85(m,2H),0.57(m,2H); m / z(MS-ESI):254.47[M+1] + .
[0043] The synthetic conditions of 2-hydroxymethyl-3-cyclopropylamino-6-methylthiopyrazine 3 are:
[0044] Mix ethyl 3-cyclopropylamino-6-methylthio-2-pyrazinecarboxylate 2 (31.5g), lithium aluminum hydride (14.2g) and tetrahydrofuran (150mL), cool to -60-0°C, stir 2-5 hours; warm up to room temperature, add saturated ammo...
Embodiment 2
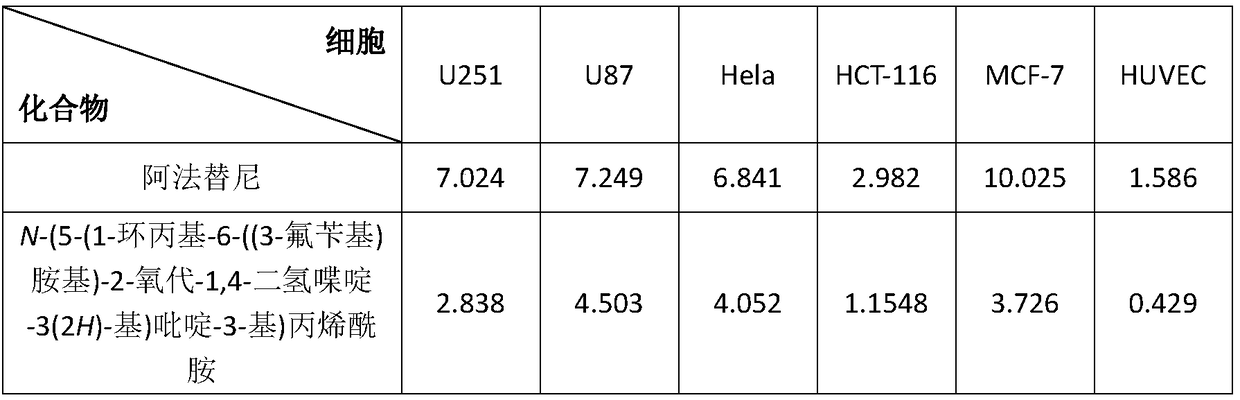
[0062] Biological Activity of Compounds of the Invention
[0063] 1. Experimental materials
[0064] 1. Cell lines: human glioma cell U251, human brain astroglioblastoma cell U87, cervical cancer cell Hela, human colon cancer cell HCT-l16, human breast cancer cell line MCF-7, human umbilical cord Venous endothelial cell line HUVEC.
[0065] 2. Experimental reagents: MTT (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide), PBS buffer, DMEM medium: Gibco, fetal bovine serum: Sijiqing company, DMSO: Sigma, trypsin: Gibco.
[0066] 3. Experimental equipment: cell incubator: Japan Sanyo Electric Co., Ltd., microplate reader: MOLECULAR company, optical microscope: LEICA company, ultra-clean workbench: Beijing Weida Purification Technology Research Institute.
[0067] Second, the preparation of reagents:
[0068] (1) PBS solution: NaCl 8g, KCl 0.2g, NaCl 2 HPO 4 2H 2 O 3.62g, KH 2 PO 4
[0069] 0.24g, the above mixture was dissolved in 900ml of distilled water, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com