Tumor-targeting near-infrared fluorescent probe and preparation method thereof
A technology of tumor-targeting and fluorescent probes, which is applied in the field of tumor-targeting near-infrared fluorescent probes and its preparation, can solve problems such as lack of selectivity, unfavorable detection and analysis, and inability to achieve specific labeling of target proteins. The effect of improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Synthesis of 1-(4-sulfobutyl)-2,3,3-trimethyl-3H-indole
[0022] Take 4.8 g of 2,3,3-trimethyl-3H-indole and 13.6 g of 1,4-butane sultone, add 40 ml of 1,2-dichlorobenzene, and heat to 120°C under nitrogen to react 8 hours, then cooled to room temperature, added 200 ml of acetone to precipitate, filtered, added an appropriate amount of methanol, and then added isopropanol to precipitate to obtain 1-(4-sulfobutyl)-2,3,3-trimethyl-3H- indole.
Embodiment 2
[0023] Example 2: Synthesis of 5-carboxy-1-(4-sulfobutyl)-2,3,3-trimethyl-3H-indole
[0024] Take 4.56 grams of hydrazinobenzoic acid, 5 grams of sodium acetate and 3.7 grams of methyl isopropyl ketone, dissolve them in 30 milliliters of glacial acetic acid, stir and react at room temperature for 1 hour, then heat to 50 ° C for 4.5 hours, and cool to room temperature. Remove the solvent by rotary evaporation under reduced pressure, wash with 20 ml of water and 2 ml of methanol, dissolve the precipitate in 20 ml of 1,2-dichlorobenzene, add 4.3 g of 1,4-butane sultone, resuspend, and heat to React at 180°C for 5 hours, then cool to room temperature, filter, wash with an appropriate amount of acetone, and dry to obtain 5-carboxy-1-(4-sulfobutyl)-2,3,3-trimethyl-3H-indole.
Embodiment 3
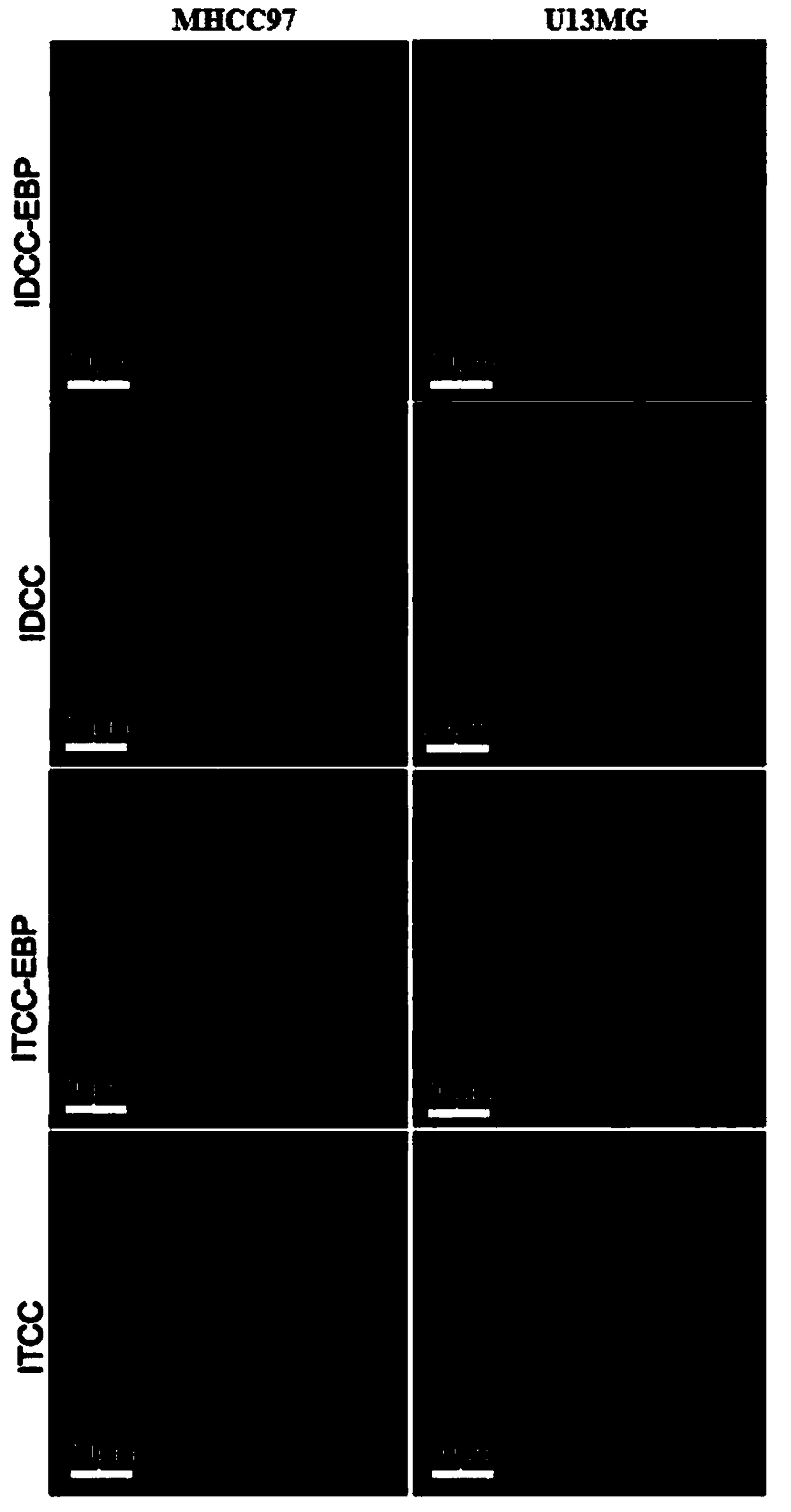
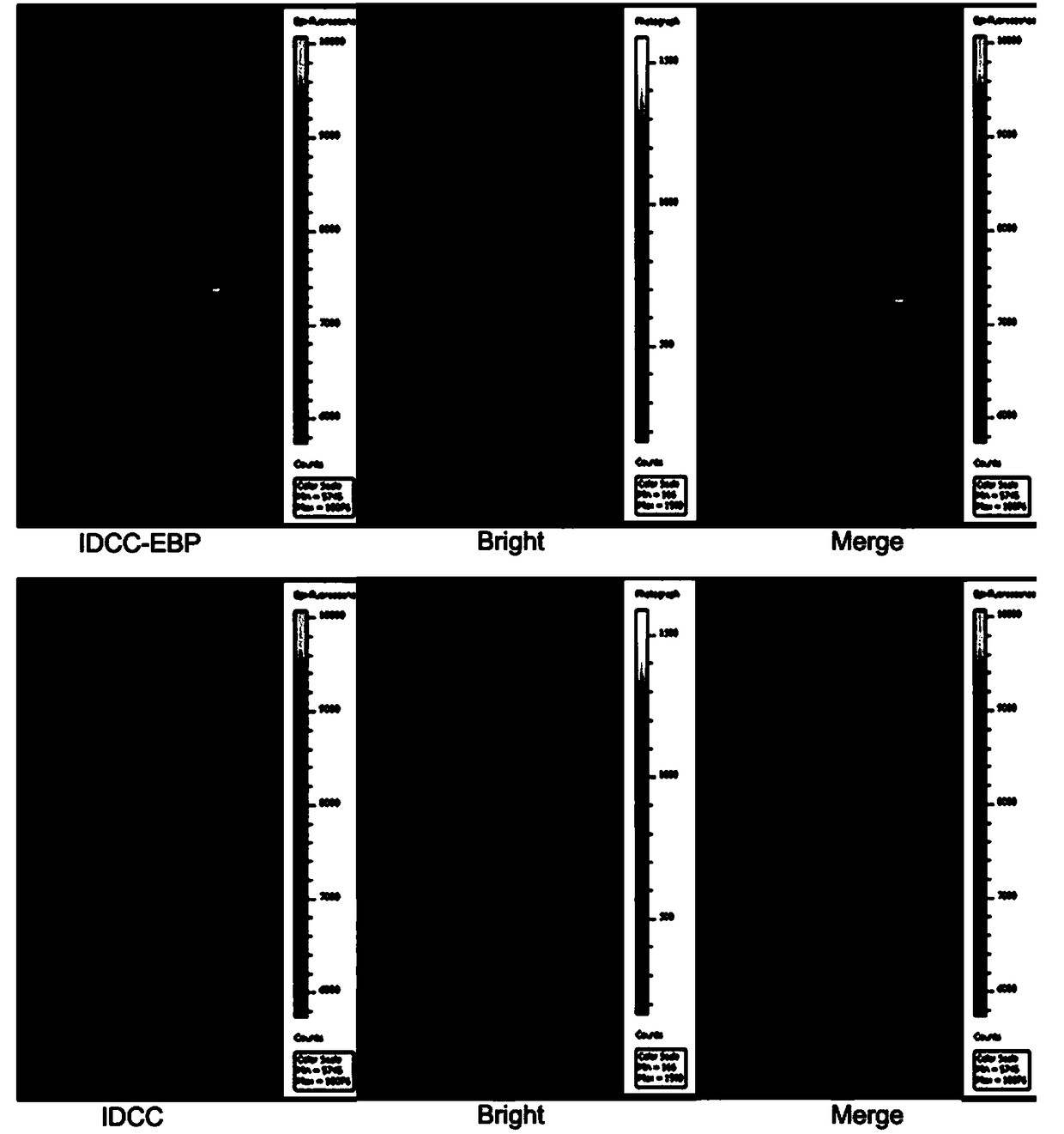
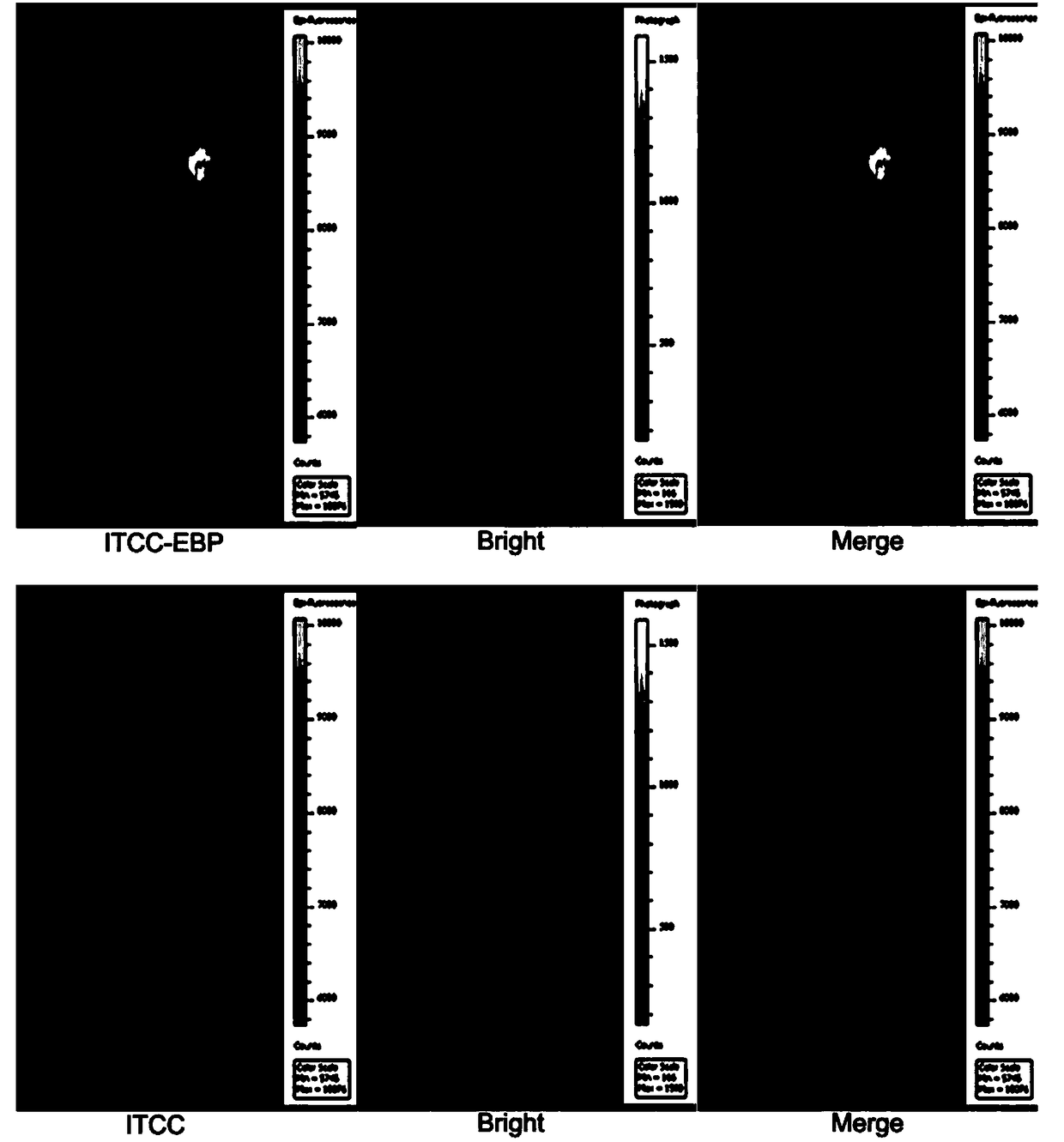
[0025] Example 3: Synthesis of 5-carboxylic acid-1,1'-bis-(4-sulfobutyl) sodium indole dicarbocyanine (IDCC)
[0026] Weigh 1 g of 1-(4-sulfobutyl)-2,3,3-trimethyl-3H-indole and 0.78 g of malondialdehyde bisphenylimide monohydrochloride, dissolve in 12 ml of anhydrous acetic acid , heated to 120°C and stirred for 30 minutes, cooled to room temperature and added 1.2 grams of 5-carboxy-1-(4-sulfobutyl)-2,3,3-trimethyl-3H-indole and 1 gram of acetic acid sodium, plus 12 ml of anhydrous acetic acid, heated to 120 ° C and stirred, cooled to room temperature, extracted with 150 ml of ether, filtered, purified by reverse chromatography, and freeze-dried to obtain 5-carboxylic acid-1,1'-bis -(4-sulfobutyl)indole dicarbocyanine sodium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com