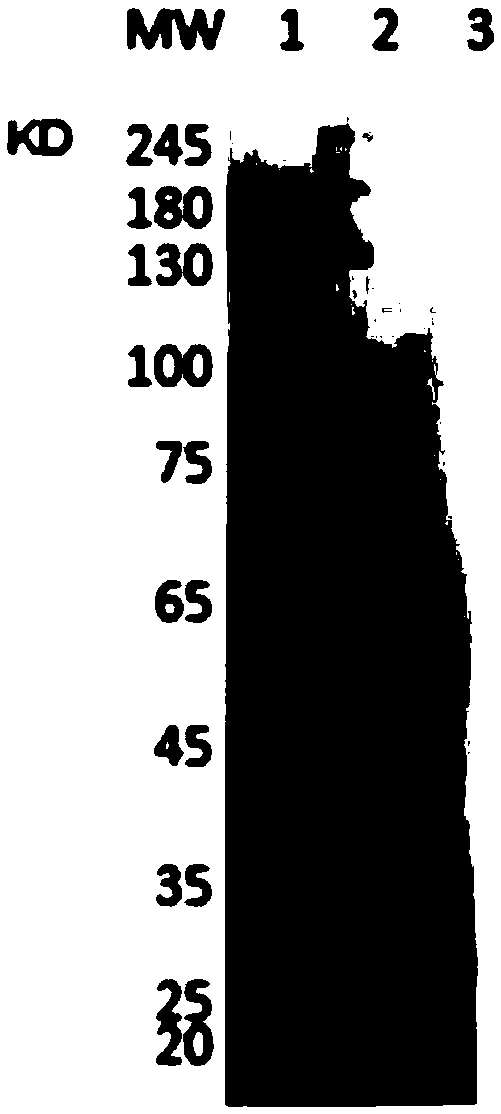
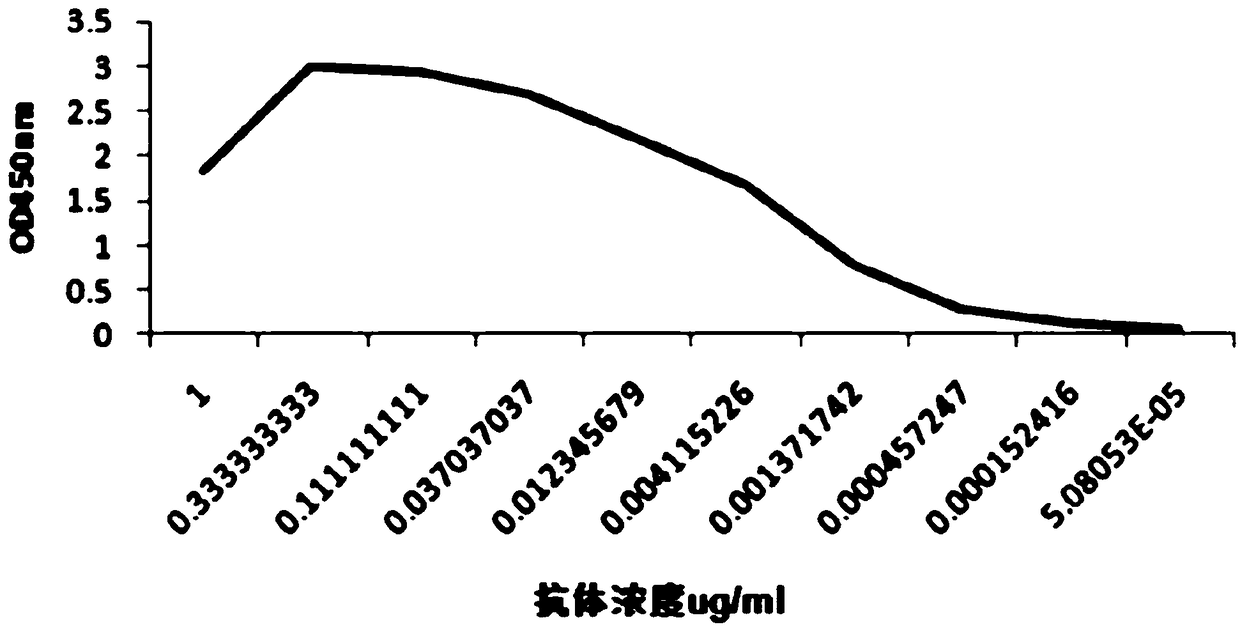
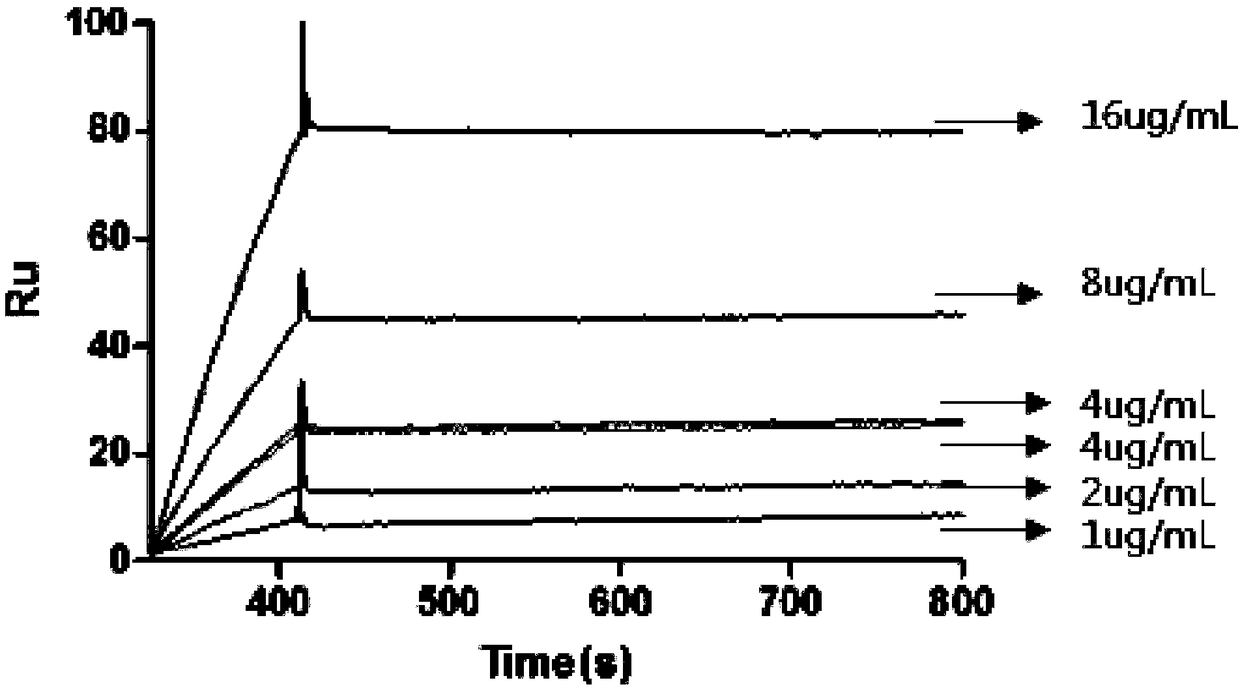
Antitetanus toxin neutralizing antibody as well as preparation method and application thereof
An antibody and antigen technology, applied in the field of cellular immunology, can solve the problems of unresolved industrial production of antibodies and low titer of neutralizing toxins, and achieve the effect of controllable standards and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Mononuclear Cell Separation and Plasma Cell Sorting
[0069] (1) Collect 15mL blood samples from the blood of healthy volunteers injected with 1500IU tetanus toxoid vaccine on the 0th, 14th and 21st days respectively, put them in anticoagulant tubes containing heparin, separate them with Ficoll, and absorb mononuclear cells ( PBMC) layer suspension was washed 3 times with PBS, and the supernatant was discarded by suction;
[0070](2) Filter with a 40 μm filter membrane, count the cells, and use the BD FACSria flow cytometer to sort out the target cell population from the PBMC, and select a single cell with a good shape and place it in a 96-well PCR (Polymerase Chain Reaction, polymerase chain reaction) Plate (20 μL single-cell lysate per well), so that each well contains one B cell, and store in a -80°C refrigerator for later use.
Embodiment 2
[0071] Example 2 Isolation of Antibody Variable Region Genes from Single B Cells Using RT-PCR
[0072] (1) RT-PCR: Add 0.5 μM constant region primers of heavy and light chains of different subtypes to a 96-well plate containing a single B cell (primers are designed at specific sites by conventional methods) and Superscript III reverse transcriptase , incubate at 37°C for 1 hour, and carry out PCR amplification according to the following conditions: 95°C for 15 min; 95°C for 1 min, 55°C for 1 min, 72°C for 1 min, 30 cycles; 72°C for 10 min; 4°C for 5 min; the obtained product cDNA was stored at -20 Store at ℃;
[0073] (2) PCR: 5 μL of reverse transcription product, HotStarTaq Plus enzyme, dNTPs, and 0.5 μM specific primers for the variable regions of heavy and light chains of different subtypes in a 50 μL system (primers were designed at specific sites by conventional methods) ), carry out PCR amplification according to the following conditions: pre-denaturation at 94°C for 5...
Embodiment 3
[0075] Embodiment 3 constructs the expression vector of recombinant antibody
[0076] The PCR product of the variable region gene of the antibody identified as positive by gel electrophoresis and whose heavy chain and light chain can be paired is connected to the pcDNA3.3 vector by TA cloning method to construct a fully human neutralized anti-tetanus toxin The expression vector of the antibody, and then transform the expression vector into DH5α competent bacteria, culture on a plate containing ampicillin at 37°C overnight, pick 10 single colonies and perform PCR with specific primers, the reaction conditions are: 94°C pre-denaturation for 3 minutes; Denaturation at 94°C for 30s, annealing at 55°C for 30s, extension at 72°C for 100s, 28 cycles; extension at 72°C for 5min. Take 5 μL of PCR products and detect them by 1% agarose gel electrophoresis.
[0077] The results showed that transformants containing antibody heavy chain and light chain genes were identified among the posi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com