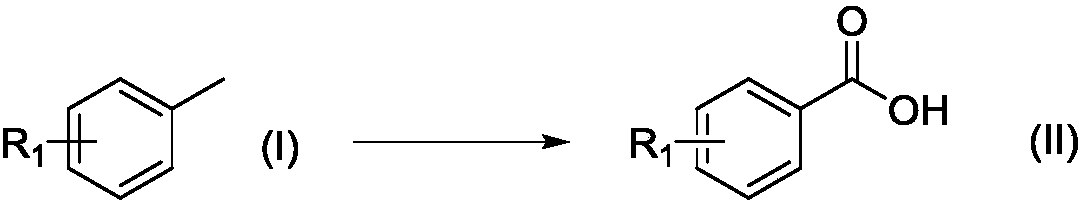
Method for preparing aryl formic acid compound with microchannel continuous flow reactor
A technology of aryl formic acid and microchannel, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylate, etc., can solve problems such as potential safety hazards, achieve safety assurance, small liquid holding capacity, and reduce environmental toxicity The effect of the formation of harmful by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
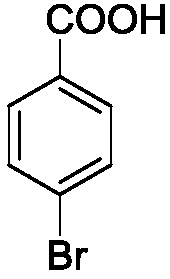
[0025] Example 1: Preparation of p-bromobenzoic acid
[0026]
[0027] P-bromotoluene (Formula I, R=4-Br) 1.71g (10mmol), cobalt naphthenate 0.0156g (0.05mmol), manganese acetate 0.0087g (0.05mmol), iron nitrate 0.0404g (0.1mmol), 30 % mass concentration hydrogen peroxide 0.034g (1mmol), acetonitrile 30ml, mix well. Mix this solution with 0.2L / min O in a mixer at a flow rate of 0.5ml / min 2 (O 2 Pressure is 2MPa) mixed and flows through the microreactor (the microreactor has 20 microchannel mixer substrates) heated to 100 ℃ in advance, the reaction residence time is 47.89s, and the reaction mixture flows into the beaker through the back pressure valve, Sodium sulfite was added to remove excess hydrogen peroxide, and the solvent was distilled off under reduced pressure to obtain 1.42 g of p-bromobenzoic acid with a yield of 83%.
[0028] Proton NMR spectrum: (400MHz, Chloroform-d) δ13.205(s, 1H), 7.905(d, J=8Hz, 2H), 6.97(d, J=8Hz, 2H)
example 2
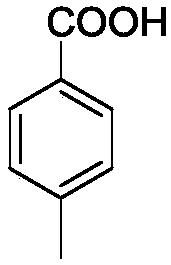
[0029] Example 2: Preparation of p-toluic acid
[0030]
[0031] P-methyltoluene (Formula I, R=4-CH 3 ) 1.06g (10mmol), cobalt stearate 0.1251g (0.2mmol), manganese acetate 0.0346g (0.2mmol), ferric chloride 0.0811g (0.5mmol), 30% mass concentration hydrogen peroxide 1.02g (3mmol), acetic acid 25ml, mix well. Mix this solution with 0.4L / min O in a mixer at a flow rate of 1ml / min 2 (O 2 The pressure is 10MPa) mixed and flows through the microreactor (the microreactor has 35 microchannel mixer substrates) preheated to 120°C, the reaction residence time is 42s, the reaction mixture flows into the beaker through the backup valve, and cools , solids were precipitated, and filtered by suction to obtain 0.99 g of p-toluic acid with a yield of 93%.
[0032] Proton NMR spectrum: (400MHz, Chloroform-d) δ12.830(s, 1H), 7.875(d, J=8Hz, 2H), 7.318(d, J=8Hz, 2H), 2.377(s, 1H),
example 3
[0033] Example 3: Preparation of m-methoxybenzoic acid
[0034]
[0035] 1.22g (10mmol) of m-methyltoluene (Formula I, R=3-OMe), 0.0125g (0.05mmol) of cobalt acetate, 0.0173g (0.1mmol) of manganese acetate, 0.1398g (0.3mmol) of ferric oxalate pentahydrate, 0.034g (1mmol) of 30% mass concentration hydrogen peroxide and 10ml of acetonitrile were mixed uniformly. Put this solution in a mixer at a flow rate of 0.5ml / min at 0.1L / min O 2 (O 2 The pressure is 0.5MPa) mixed and flowed through the microreactor preheated to 25°C (the microreactor has a microchannel mixer substrate), the reaction residence time is 4.78s, and the reaction mixture flows into the beaker through the back pressure valve , adding sodium sulfite to remove excess hydrogen peroxide, and distilling off the solvent under reduced pressure to obtain 0.7686 g of m-methoxybenzoic acid with a yield of 63%.
[0036] Proton NMR spectrum: (400MHz, Chloroform-d) δ9.973(s, 1H), 7.468(d, J=8.1Hz, 2H), 7.388(d, J=8.0Hz, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com