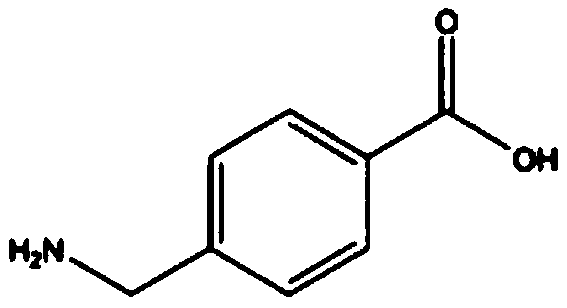
Synthetic method for aminomethylbenzoic acid
A technique for the synthesis of aminotoluic acid, which is applied in chemical instruments and methods, the preparation of organic compounds, organic chemistry, etc., can solve problems such as environmental pollution and severe toxicity, and achieve simple and easy-to-obtain raw materials, accelerated reaction rates, and high yields. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
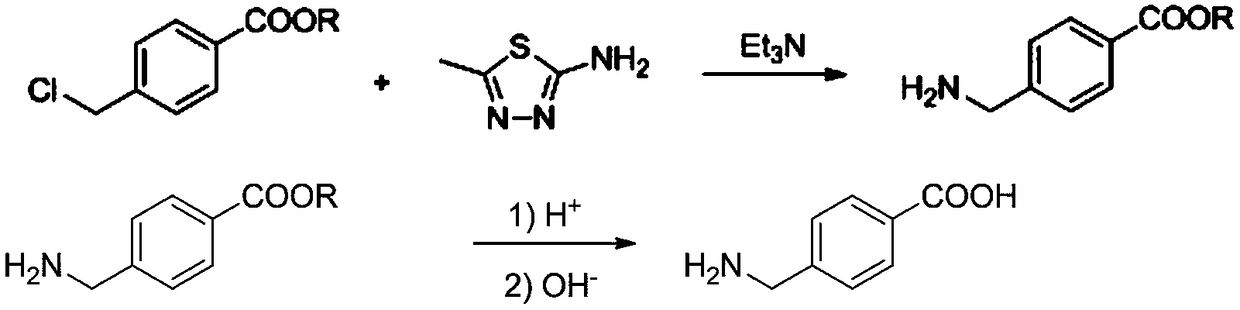
[0038] 1) Add methyl 4-chloromethylbenzoate and triethylamine to the reaction vessel, and then add 2mol / L of 2-amino-5-methyl-1,3,4-thiadiol dropwise under stirring Ethanol-water solution of oxadiazole (mass ratio of ethanol and water: 10:1), react at 100°C for 0.5h, after the reaction is complete, evaporate the solution until a large amount of solids are precipitated, cool, filter, and dry to obtain white solid 4-aminomethyl methyl benzoate; wherein, the molar ratio between methyl 4-chloromethylbenzoate, triethylamine and 2-amino-5-methyl-1,3,4-thiadiazole is 1:1.2: 1;
[0039] 2) Add methyl 4-aminomethylbenzoate to concentrated sulfuric acid solution, the molar ratio of sulfuric acid and 4-aminomethylalkylbenzoate is 1:1, stir and react at 50°C for 1.5h, and react After finishing, be cooled to room temperature, add water (the amount of water substance is 20 times of the amount of 4-aminomethylbenzoic acid methyl ester substance), then add sodium hydroxide solution drop by d...
Embodiment 2
[0041] 1) Add ethyl 4-chloromethylbenzoate and triethylamine to the reaction vessel, then add 2mol / L of 2-amino-5-methyl-1,3,4-thiadiol dropwise under stirring Ethanol-water solution of oxadiazole (mass ratio of ethanol and water: 10:1), react at 80°C for 1.5h, after the reaction is complete, evaporate the solution until a large amount of solids are precipitated, cool, filter, and dry to obtain white solid 4-aminomethyl ethyl benzoate; wherein, the molar ratio between ethyl 4-chloromethylbenzoate, triethylamine and 2-amino-5-methyl-1,3,4-thiadiazole is 1:1.5: 1;
[0042]2) Add ethyl 4-aminomethylbenzoate to the concentrated sulfuric acid solution, the molar ratio of sulfuric acid to 4-aminomethylalkylbenzoate is 2:1, stir and react at 80°C for 1h, and the reaction ends Afterwards, be cooled to room temperature, add water (the amount of water substance is 20 times of the amount of 4-aminomethylbenzoic acid methyl ester substance), then add ammoniacal liquor drop by drop under ...
Embodiment 3
[0044] 1) Add ethyl 4-bromomethylbenzoate and triethylamine to the reaction vessel, then add 2mol / L of 2-amino-5-methyl-1,3,4-thiadiol dropwise under stirring Ethanol-water solution of oxadiazole (mass ratio of ethanol and water: 10:1), react at 70°C for 3 hours, after the reaction is complete, evaporate the solution until a large amount of solids are precipitated, cool, filter, and dry to obtain 4-aminomethyl as a white solid Ethyl benzoate; wherein the molar ratio between ethyl 4-bromomethylbenzoate, triethylamine and 2-amino-5-methyl-1,3,4-thiadiazole is 1:2:1 ;
[0045] 2) Add ethyl 4-aminomethylbenzoate to concentrated sulfuric acid solution, the molar ratio of sulfuric acid and 4-aminomethylalkylbenzoate is 3:1, stir and react at 90°C for 0.5h, and react After finishing, be cooled to room temperature, add water (the amount of water substance is 20 times of the amount of 4-aminomethylbenzoic acid methyl ester substance), then add ammoniacal liquor drop by drop under stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com