A high ionic conductivity solid-state electrolyte, its preparation method and its application in all-solid-state lithium ion batteries
A lithium ion battery and conductivity technology, which is applied to the high ionic conductivity solid electrolyte, the application of the high ionic conductivity solid electrolyte in the all-solid lithium ion battery, the preparation of the high ionic conductivity solid electrolyte, and can solve the interface impedance Large, large battery internal resistance, solid-state batteries do not have much competitive advantages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
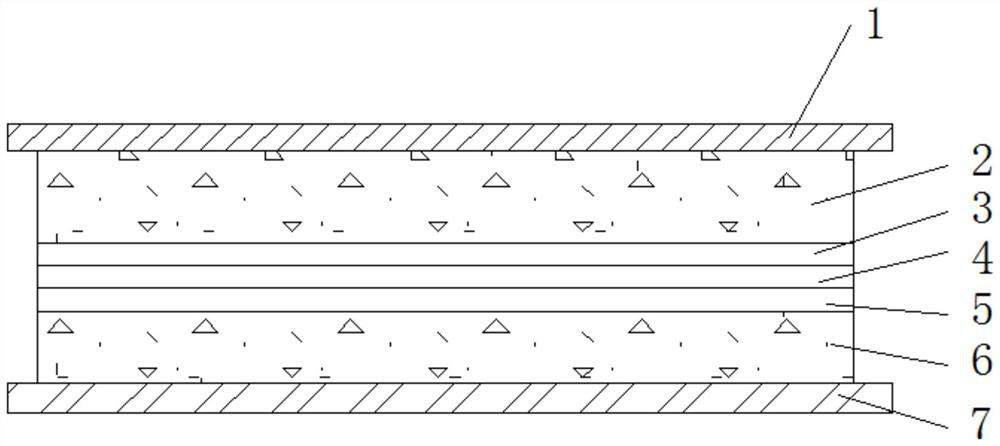
[0020] A solid electrolyte with high ionic conductivity, the solid electrolyte layer includes lithium salt, ceramic powder, adhesive and solvent, the mass ratio of lithium salt, ceramic powder, adhesive and solvent is 10:1:10:79 ;
[0021] The lithium salt is one or more of lithium hexafluorophosphate, lithium bistrifluoromethanesulfonylimide, lithium perchlorate, lithium tetrafluoroborate and lithium dioxalate borate, preferably bistrifluoromethanesulfonylimide lithium.
[0022] The ceramic particles include one or more of LiLaZrO, LiLaTiO, LiLaZrTaO, preferably LiLaZrO.
[0023] The adhesive is composed of one or more of polyacrylonitrile, polypropylene oxide, polysiloxane, polyvinylidene fluoride, polymethyl methacrylate and polyvinylidene fluoride-hexafluoropropylene , preferably polyvinylidene fluoride and polyvinylidene fluoride-hexafluoropropylene, wherein the mass ratio of polyvinylidene fluoride to polyvinylidene fluoride-hexafluoropropylene is 0.5:9.5.
[0024] Th...
Embodiment 2
[0033] A solid electrolyte with high ion conductivity, the solid electrolyte layer includes lithium salt, ceramic powder, adhesive and solvent, the mass ratio of lithium salt, ceramic powder, adhesive and solvent is 2:4:2:90 ;
[0034] The lithium salt is one or more of lithium hexafluorophosphate, lithium bistrifluoromethanesulfonimide, lithium perchlorate, lithium tetrafluoroborate and lithium dioxalate borate, preferably lithium hexafluorophosphate.
[0035] The ceramic particles include one or more of LiLaZrO, LiLaTiO, and LiLaZrTaO, preferably LiLaTiO.
[0036] The adhesive is composed of one or more of polyacrylonitrile, polypropylene oxide, polysiloxane, polyvinylidene fluoride, polymethyl methacrylate and polyvinylidene fluoride-hexafluoropropylene , preferably polymethyl methacrylate.
[0037] The solvent is one of N-methylpyrrolidone, dimethylformamide, ethanol, ethyl acetate, preferably dimethylformamide.
[0038] A method for preparing a high-ionic-conductivity ...
Embodiment 3
[0046] A solid electrolyte with high ionic conductivity, the solid electrolyte layer includes lithium salt, ceramic powder, adhesive and solvent, the mass ratio of lithium salt, ceramic powder, adhesive and solvent is 20:10:20:50 ;
[0047] The lithium salt is one or more of lithium hexafluorophosphate, lithium bistrifluoromethanesulfonimide, lithium perchlorate, lithium tetrafluoroborate and lithium dioxalate borate, preferably lithium tetrafluoroborate.
[0048] The ceramic particles include one or more of LiLaZrO, LiLaTiO, LiLaZrTaO, preferably LiLaZrTaO.
[0049] The adhesive is composed of one or more of polyacrylonitrile, polypropylene oxide, polysiloxane, polyvinylidene fluoride, polymethyl methacrylate and polyvinylidene fluoride-hexafluoropropylene , preferably polypropylene oxide.
[0050] The solvent is one of N-methylpyrrolidone, dimethylformamide, ethanol and ethyl acetate, preferably ethyl acetate.
[0051] A method for preparing a high-ionic-conductivity soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com