Rare earth-modified nickel-based catalyst for pressurized carbon dioxide reforming of methane to synthesis gas
A nickel-based catalyst and carbon dioxide technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, heterogeneous catalyst chemical elements, etc., can solve the problem that the reforming methane reaction cannot continue and is difficult to achieve Commercial application, catalyst bed clogging and other problems, to achieve the effect of inhibiting carbon deposition, increasing dispersion and surface active metal content, and eliminating surface carbon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
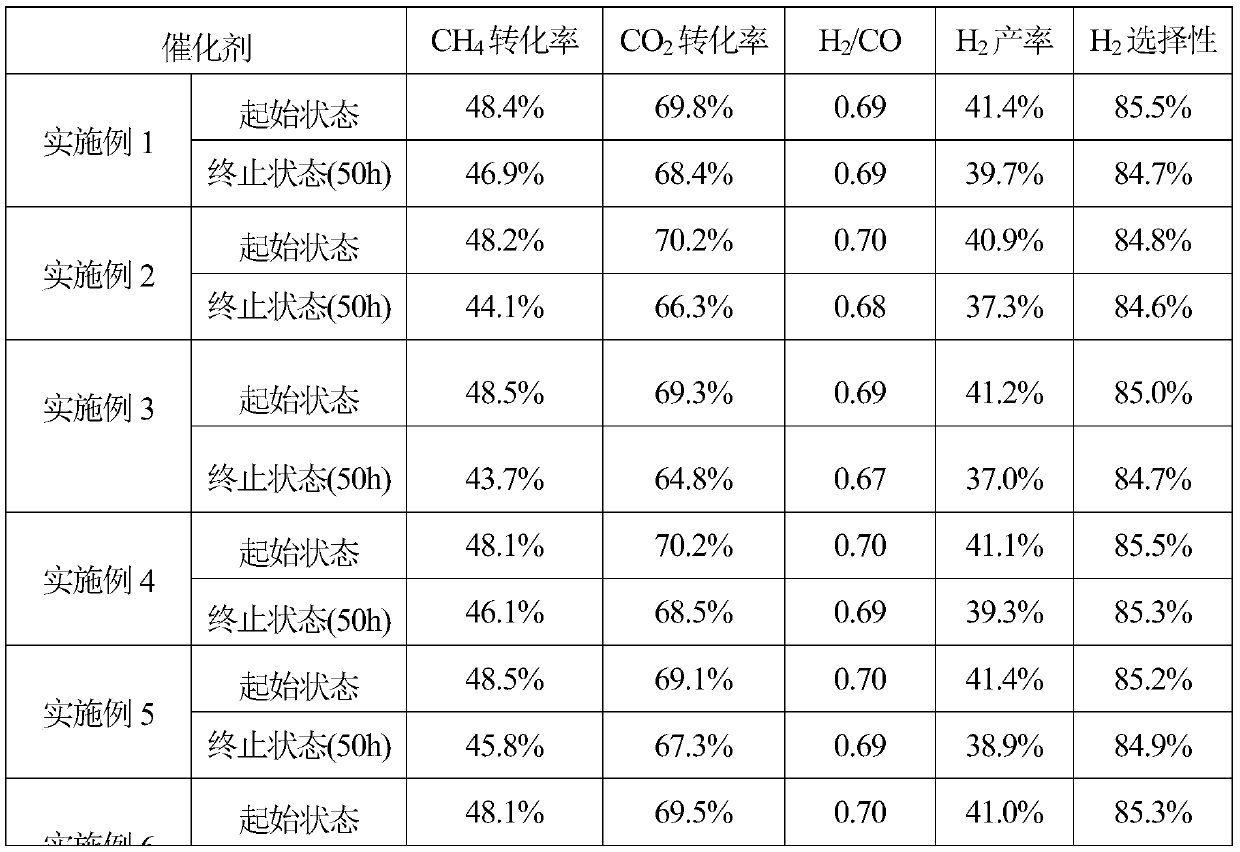
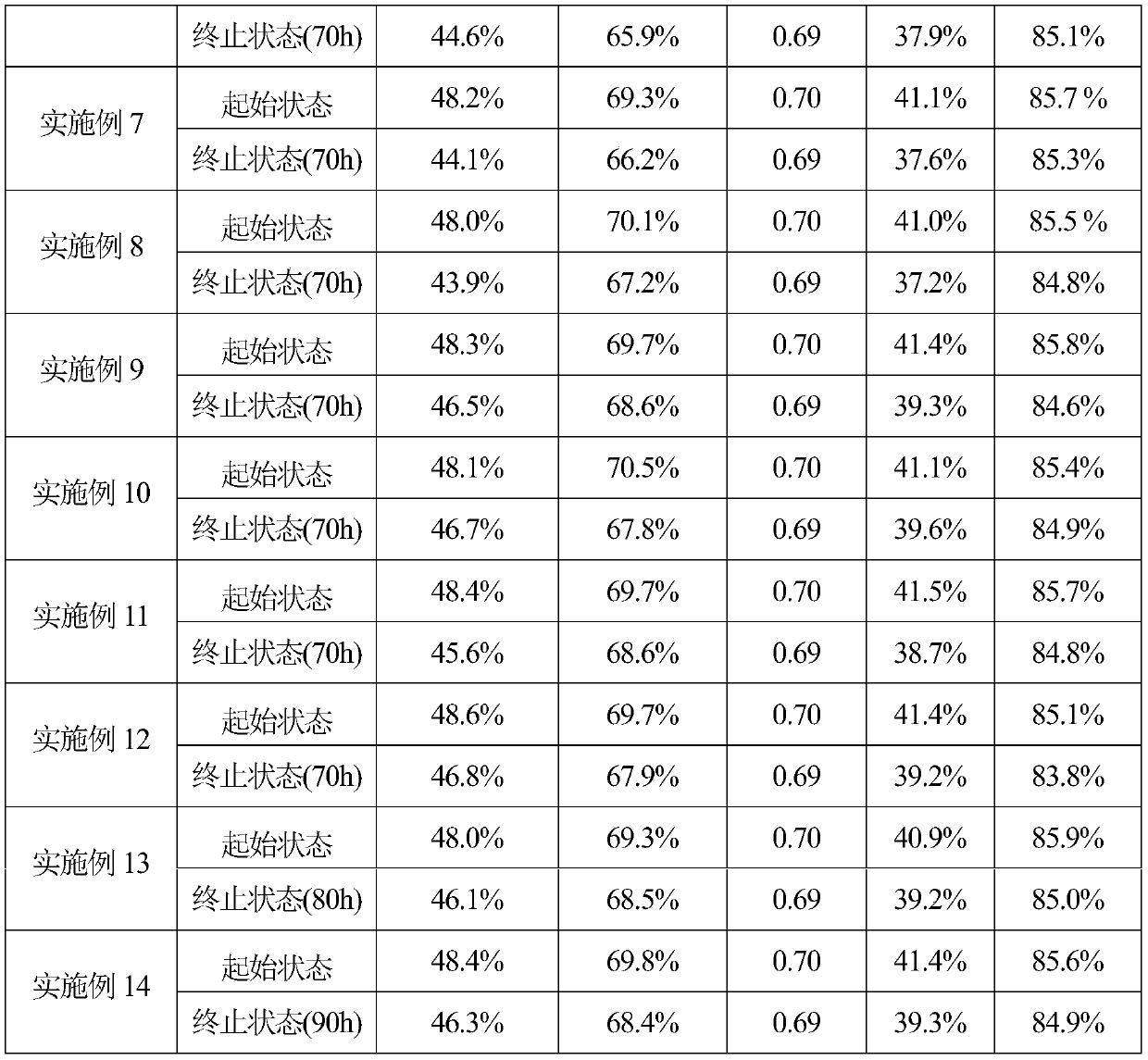
Embodiment 1
[0021] According to the catalyst composition, it is 10%Ni-6.3%La 2 o 3 -SiO 2 , 0.5509g (1.2723mmol) of lanthanum nitrate hexahydrate and 1.63g (5.6054mmol) of nickel nitrate hexahydrate and 9.5482g (45.831mmol) of ethyl orthosilicate were dissolved in 40g of ethanol to obtain solution A. Then 6.6444g (52.708mmol) of oxalic acid dihydrate was dissolved in 40g of distilled water to obtain solution B. Add solution B to solution A, stir at room temperature for 4 hours, evaporate the solvent with a rotary evaporator to obtain a viscous liquid, move it to an electric furnace for combustion, and place the solid powder obtained by combustion in a muffle furnace for roasting. The heating rate of ℃ / min is raised to 700 ℃, kept at constant temperature for 4 hours, naturally cooled to room temperature, taken out, pressed into tablets, granulated, passed through a 40-60 mesh sieve, and prepared into a catalyst.
Embodiment 2
[0023] 10%Ni-5.0%Sm according to catalyst composition 2 o 3 -SiO 2 , 0.4193g (0.9434mmol) samarium nitrate hexahydrate, 1.63g (5.6054mmol) nickel nitrate hexahydrate and 9.6965g (46.5437mmol) ethyl orthosilicate were dissolved in 40g ethanol to obtain solution A. Then 11.1568g (53.0921mmol) of citric acid monohydrate was dissolved in 40g of distilled water to obtain solution B. Add solution B to solution A, stir at room temperature for 4 hours, evaporate the solvent with a rotary evaporator to obtain a viscous liquid, move it to an electric furnace for combustion, and place the solid powder obtained by combustion in a muffle furnace for roasting. The heating rate of ℃ / min is raised to 700 ℃, kept at constant temperature for 4 hours, naturally cooled to room temperature, taken out, pressed into tablets, granulated, passed through a 40-60 mesh sieve, and prepared into a catalyst.
Embodiment 3
[0025] 10%Ni-6.3%Pr according to catalyst composition 6 o 11 -SiO 2 , 0.5296g (1.2174mmol) of praseodymium nitrate hexahydrate, 1.63g (5.6054mmol) of nickel nitrate hexahydrate and 9.5482g (45.831mmol) of ethyl orthosilicate were dissolved in 40g of ethanol to obtain solution A. Then 5.9290 g (52.6534 mmol) of glycine was dissolved in 40 g of distilled water to obtain solution B. Add solution B to solution A, stir at room temperature for 4 hours, evaporate the solvent with a rotary evaporator to obtain a viscous liquid, move it to an electric furnace for combustion, and place the solid powder obtained by combustion in a muffle furnace for roasting. The heating rate of ℃ / min is raised to 700 ℃, kept at constant temperature for 4 hours, naturally cooled to room temperature, taken out, pressed into tablets, granulated, passed through a 40-60 mesh sieve, and prepared into a catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com