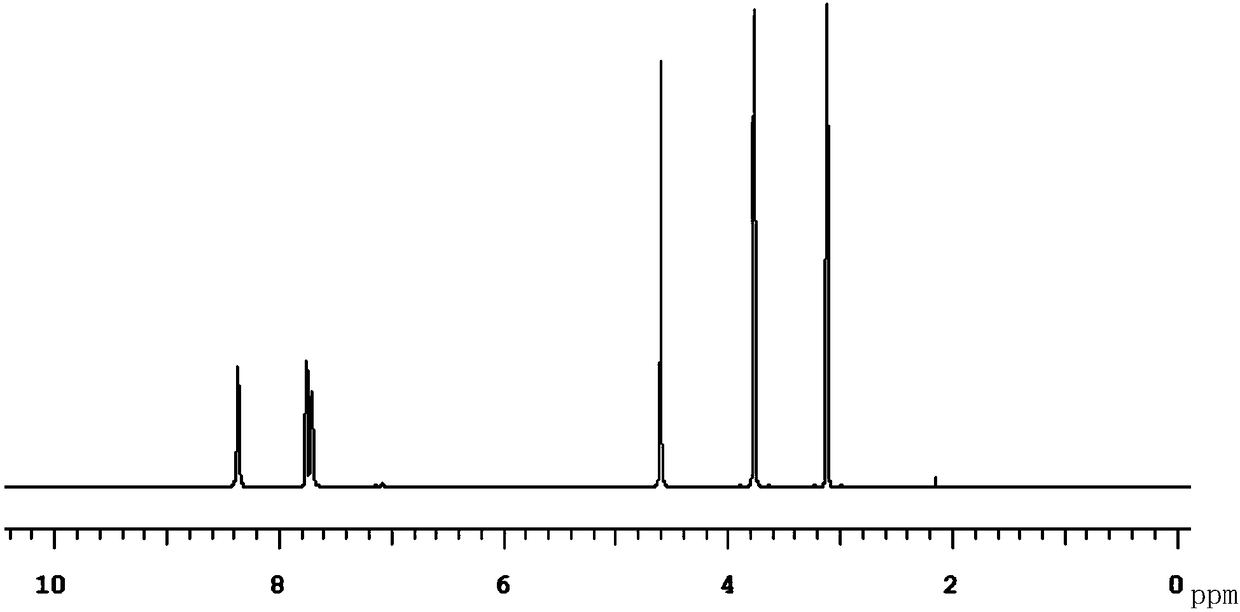
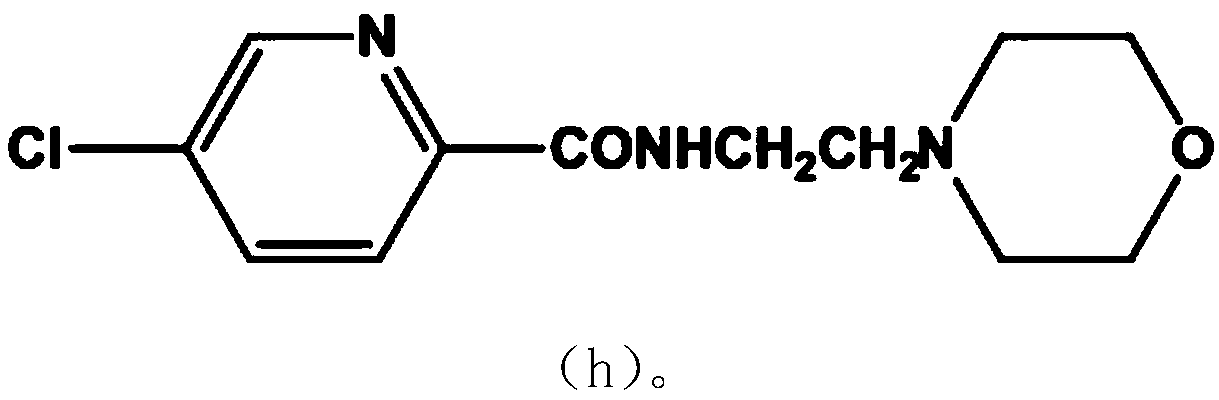
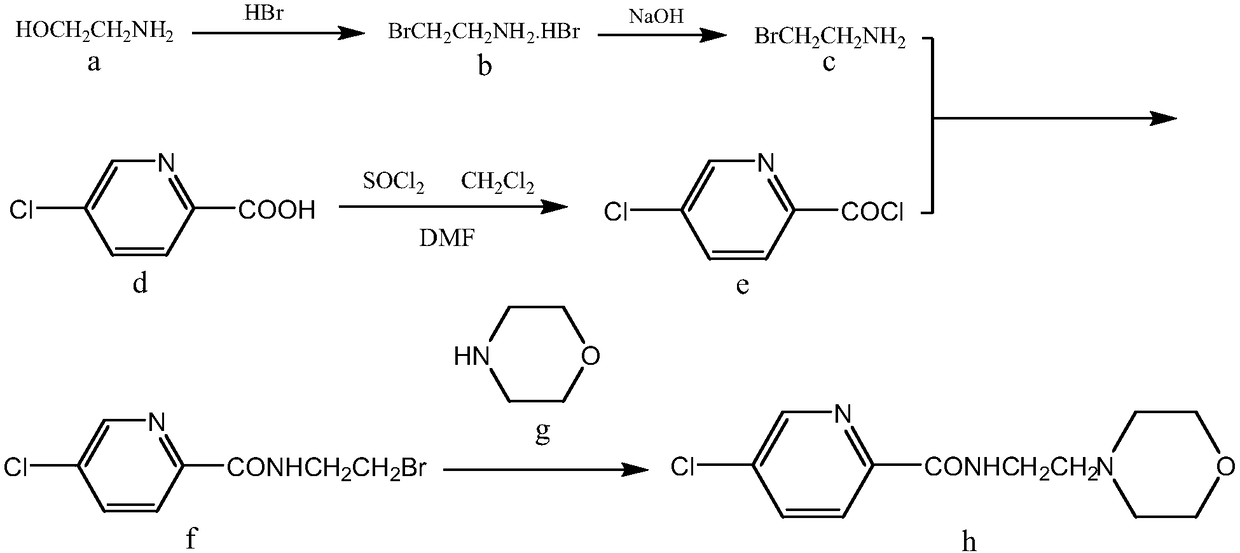
New compound Malabemide, preparation method and uses thereof
A molarabemide and compound technology, which is applied in the field of new compound morapemide, can solve the problems of difficulty in purification, toxicity, low yield and the like, and achieves the effects of simple operation, high yield and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 2-bromoethylamine hydrobromide (b)
[0026] At 0-10°C, add ethanolamine dropwise to 40% hydrobromic acid with a molar ratio of 1:9, stir magnetically, control the temperature of the reaction system at no more than 10°C, and control the time of dropping ethanolamine at 25-30min. After the dropwise addition, the solvent xylene was added to the reaction system, and the temperature was raised to separate the water for 12 hours, and the temperature of the water separation was 135-145°C. After the water separation is completed, cool, filter to remove the solvent, and wash the filter residue three times with cold acetone to obtain the white crystalline product 2-bromoethylamine hydrobromide. The yield is 99%, the purity is 99.5%, and the melting point is 172-174°C.
Embodiment 2
[0027] Example 2 Synthesis of 5-chloro-2-pyridinecarbonyl chloride (e)
[0028] At 0-10°C, first add 5-chloro-2-pyridinecarboxylic acid into the solvent dichloromethane, stir evenly with a magnetic force, then add thionyl chloride dropwise, the molar ratio is 1:4, and the temperature of the reaction system is controlled at The temperature does not exceed 10°C, and the time for dropping thionyl chloride is controlled within 15-20 minutes. After the dropwise addition, heat up and reflux for 7-8 hours. The reflux temperature is 40-45°C. After the reflux is completed, the solvent dichloromethane is removed under normal pressure, cooled, and dried in vacuum to obtain white crystals of 5-chloro-2-pyridinecarbonyl chloride . The yield is 92%, the purity is 99.4%, and the melting point is 218-220°C.
Embodiment 3
[0029] Example 3 Synthesis of 5-chloro-N-(2-bromoethyl)-2-pyridinecarboxamide (f)
[0030] First add 2-bromoethylamine hydrobromide into distilled water, and stir to dissolve with magnetic force. The sodium hydroxide solution was added dropwise to neutralize the reaction, and after the dropwise addition was completed, the solvent toluene was added, and the temperature was raised to separate water. After the water separation is completed, cool, filter, and collect the filtrate. Then add 5-chloro-2-pyridinecarbonyl chloride to the filtrate, the molar ratio is 2:1, heat up and reflux for 8-10 hours, and the reflux temperature is 115-125°C. After the reaction is completed, cool, filter with suction, and recrystallize from toluene to obtain 5-chloro-N-(2-bromoethyl)-2-pyridinecarboxamide as white crystals. The yield is 93%, the purity is 99.5%, and the melting point is 201-203°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com