Copper oxide-cobalt oxide co-doped alumina hollow sphere material and preparation method thereof
An oxide and co-doping technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve high specific surface area, simple and clear steps, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
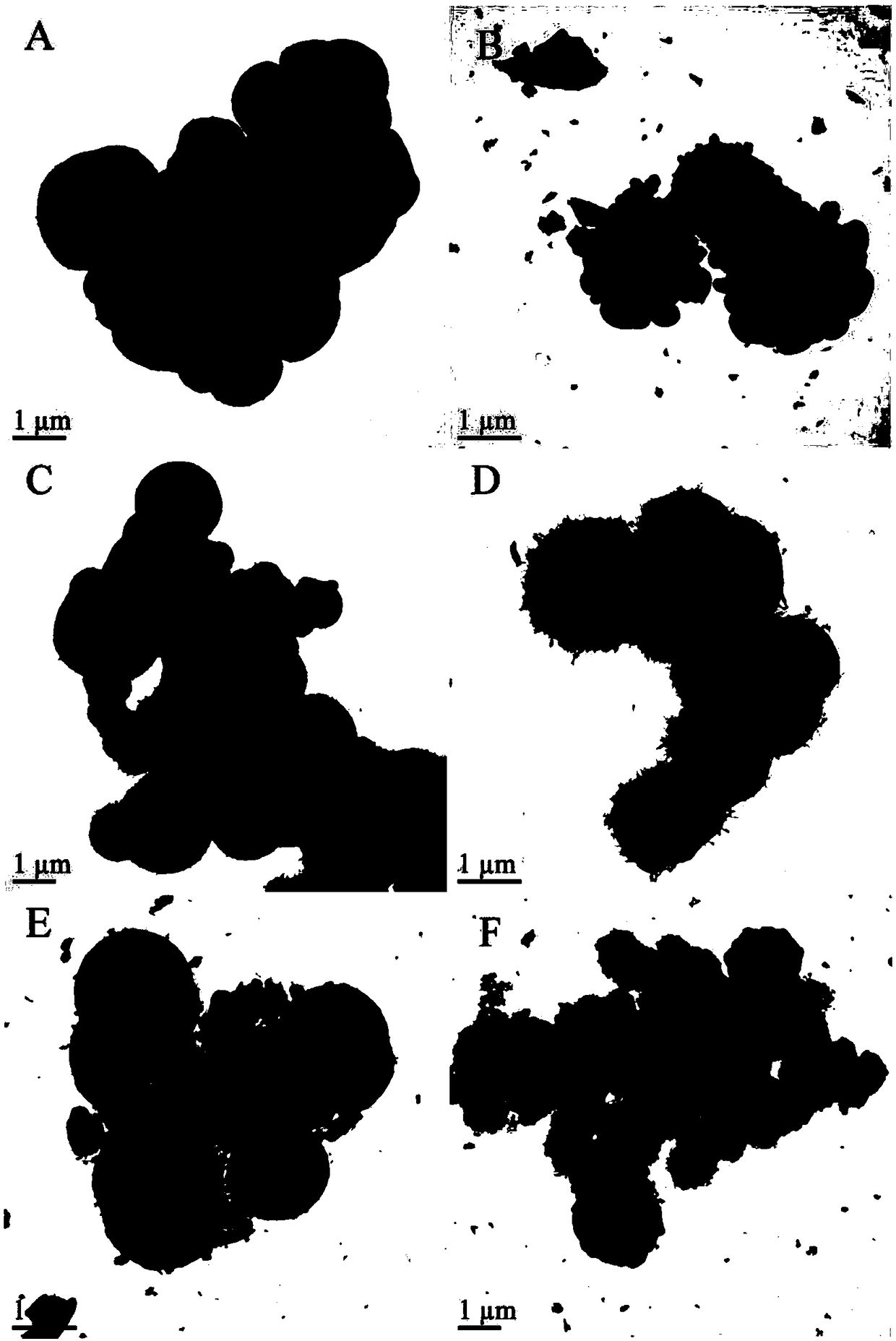
Image
Examples
Embodiment 1
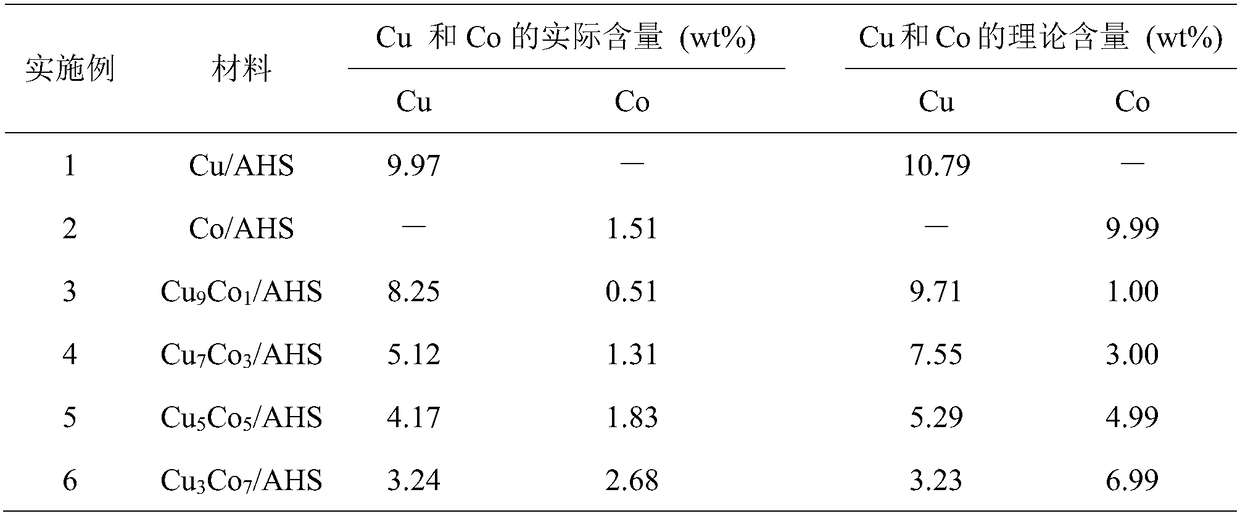
[0024] The preparation of a copper oxide-doped alumina hollow sphere material Cu / AHS in this embodiment specifically includes the following steps:
[0025] Using sucrose as sugar and carbon source, Al(NO 3 ) 3 9H 2 O is the metal precursor of aluminum, Cu(NO 3 ) 2 2.5H 2 O is the metal precursor of copper, and Cu / AHS is prepared.
[0026] 5.705g sucrose (16.67mmol), 3.126g Al(NO 3 ) 3 9H 2 O(8.33mmol) and 0.1937g Cu(NO 3 ) 2 2.5H 2 O (0.833 mmol) was co-dissolved in 25 mL of deionized water to give a clear solution. After stirring with a magnetic stirrer for 30 minutes at 25°C, the mixture was transferred to a stainless steel reaction kettle with a polytetrafluoroethylene liner, and after hydrothermal reaction at 180°C for 25 hours, it was naturally cooled to room temperature, and the obtained black The solid was washed 3 times with deionized water and absolute ethanol in sequence (the volume ratio of deionized water and absolute ethanol used each time was 2:1), dr...
Embodiment 2
[0028] The preparation of a cobalt oxide-doped alumina hollow sphere material Co / AHS in this embodiment specifically includes the following steps:
[0029] Using sucrose as sugar and carbon source, Al(NO 3 ) 3 9H 2 O is the metal precursor of aluminum, Co(NO 3 ) 2 ·6H 2 O is the metal precursor of cobalt, and Co / AHS is prepared.
[0030] 5.705g sucrose (16.67mmol), 3.126g Al(NO 3 ) 3 9H 2 O(8.33mmol) and 0.2424g Co(NO 3 ) 2 ·6H 2 O (0.833 mmol) was co-dissolved in 25 mL of deionized water to give a clear solution. After stirring with a magnetic stirrer for 40 minutes at 30°C, the mixed solution was transferred to a stainless steel reaction kettle with a polytetrafluoroethylene liner. After hydrothermal reaction at 180°C for 30 hours, it was naturally cooled to room temperature, and the obtained black The solid was washed three times with deionized water and absolute ethanol in sequence (the volume ratio of deionized water and absolute ethanol used each time was 2:1...
Embodiment 3
[0032] A kind of copper cobalt oxide doped aluminum oxide hollow sphere material Cu of the present embodiment 9 co 1 The preparation of / AHS specifically comprises the following steps:
[0033] Using sucrose as sugar and carbon source, Al(NO 3 ) 3 9H 2 O is the metal precursor of aluminum, Cu(NO 3 ) 2 2.5H 2 O and Co(NO 3 ) 2 ·6H 2 O is used as the metal precursor of copper and cobalt, respectively, to prepare Cu 9 co 1 / AHS.
[0034] 5.705g sucrose (16.67mmol), 3.126g Al(NO 3 ) 3 9H 2 O(8.33mmol), 0.1744g Cu(NO 3 ) 2 2.5H 2 O(0.750mmol) and 0.0243g Co(NO 3 ) 2 ·6H 2O (0.083mmol) was co-dissolved in 25mL deionized water to obtain a clear solution. After stirring with a magnetic stirrer for 30 minutes at 35°C, the mixed solution was transferred to a stainless steel reactor with a polytetrafluoroethylene liner. After hydrothermal reaction at 200°C for 20 hours, it was naturally cooled to room temperature, and the obtained black solid was washed with deionize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com