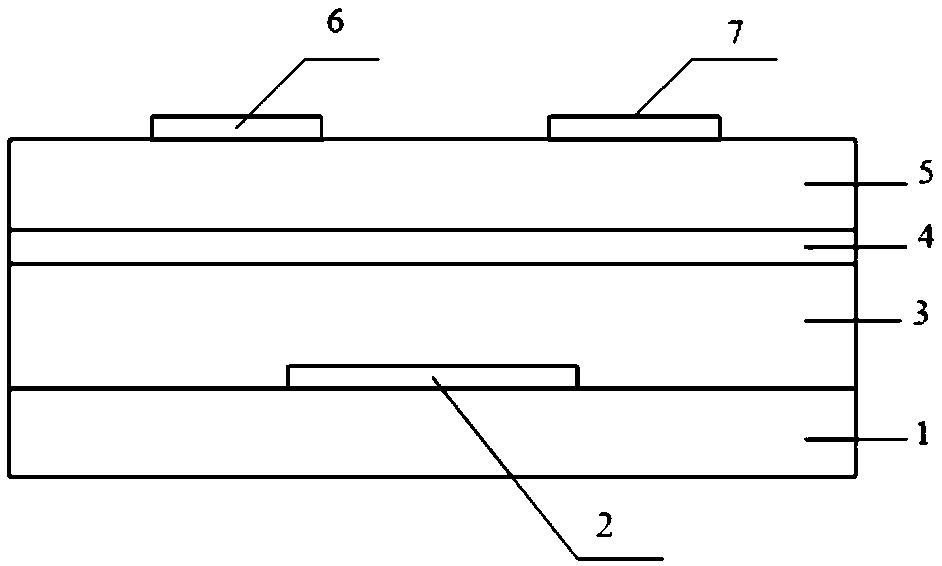
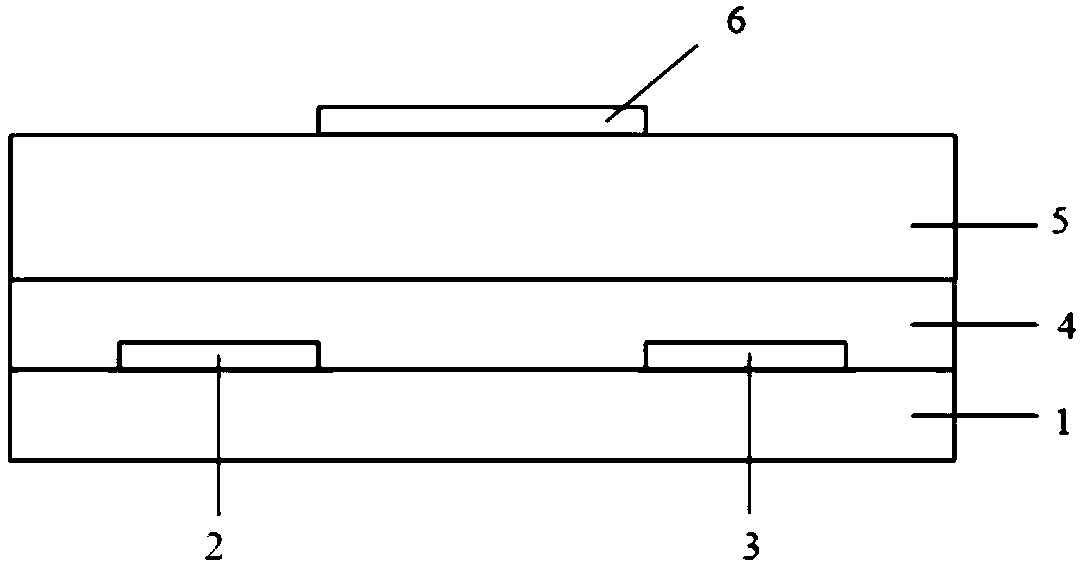
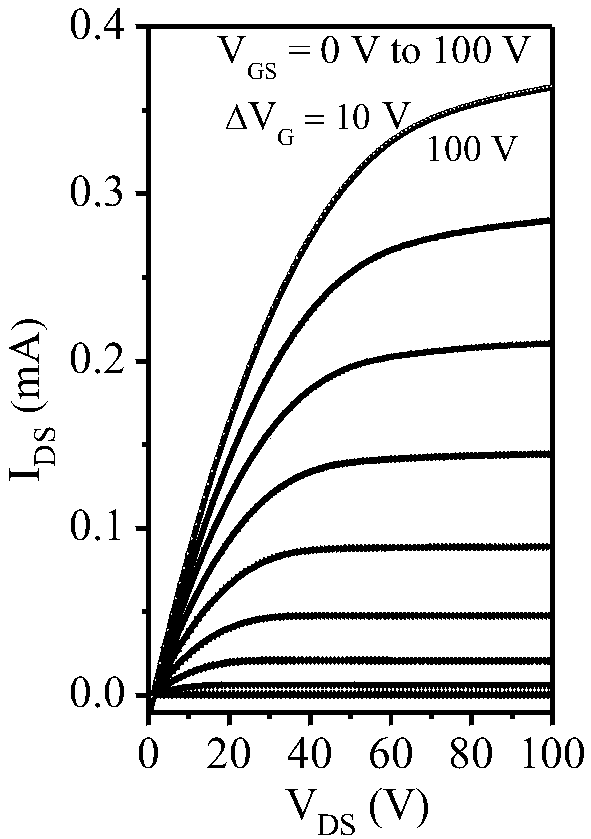
Electron-transport polymer semiconductor material based on pyridine substituted pyrrolopyrroledione and applied to organic field effect transistor
A technology for diketopyrrolopyrrole and electron transport, which is applied in silicon organic compounds, tin organic compounds, semiconductor devices, etc., and can solve the problems of difficult application of high-mobility conjugated polymers, poor reaction selectivity, and low reactivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of the polymer described in the present invention is described below. with R 1 and R 2 Taking 4-tetradecyl octadecyl as an example, the preparation process is as follows:
[0042] Step (1): 3,6-bis(5-bromopyridin-2-yl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione:
[0043]
[0044] According to the above reaction equation, it is prepared according to the method provided in the literature (Adv. Mater., 2014, 26, 2636; Adv. Funct. Mater., 2015, 25, 2725). Mix 1 times molar amount of 5-bromo-2cyanopyridine with 1 times molar amount of sodium tert-amylate and tert-amyl alcohol. After replacing the gas with high-purity argon, the temperature was raised to 110°C, and after all the solids were dissolved, 0.5 times the molar amount of diethyl succinate was added at one time, and the reaction was carried out at 110°C for 12 hours. Cool, add methanol and acetic acid in turn, filter, wash the filter cake with water and ethanol respectively, and dry to obtai...
Embodiment 1
[0070] Example 1: Preparation of 3,6-bis(5-bromopyridin-2-yl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione
[0071]
[0072] Under an inert atmosphere, 5-bromo-2cyanopyridine (9.02g, 49.2mmol) and sodium tert-amylate (5.42g, 49.2mmol) were dissolved in tert-amyl alcohol (100mL), heated to 110°C and stirred for 30min. Diethyl succinate (4.09 mL, 24.6 mmol) was added in one portion, and the reaction was stirred overnight at 110°C. After cooling down to room temperature, methanol (30 mL) was added in one portion, and after stirring for 5 min, acetic acid (30 mL) was added in one portion. The filter cake was obtained by filtration, washed with deionized water and methanol, and dried in a vacuum oven to obtain a dark red solid (6.04 g, yield 54.6%), which was directly used in the next step.
Embodiment 2
[0073] Example 2: 3,6-bis(5-bromopyridin-2-yl)-2,5-bis(2-decyltetradecyl)pyrrolo[3,4-c]pyrrole-1,4- Preparation of (2H,5 H)-diketones
[0074]
[0075] Under the protection of inert gas, 3,6-di(5-bromopyridin-2-yl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione (0.40g, 0.89 mmol), anhydrous potassium carbonate (0.37 g, 2.68 mmol) and 18-crown-6-ether (5 mg) were dissolved in dry DMF (10 mL). After fully stirring and dissolving, add 1-iodo-2-decyltetradecyl (1.23g, 2.64mmol) at one time, react at 70°C for 24 hours, distill off DMF under reduced pressure, extract the solid residue with chloroform, anhydrous sulfuric acid Magnesium was dried, filtered and spin-dried. The crude product was purified by silica gel column chromatography (eluent: petroleum ether: dichloromethane) to obtain a dark red solid (0.53 g, yield 51.3%). The structural characterization data are as follows: 1 H NMR (400MHz, CDCl 3 ,δ):8.94(d,J=8.4Hz, 2H),8.74(d,J=2.0Hz,2H),8.02(dd,J 1 =8.4Hz,J 2 =2.0Hz,2H),4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com