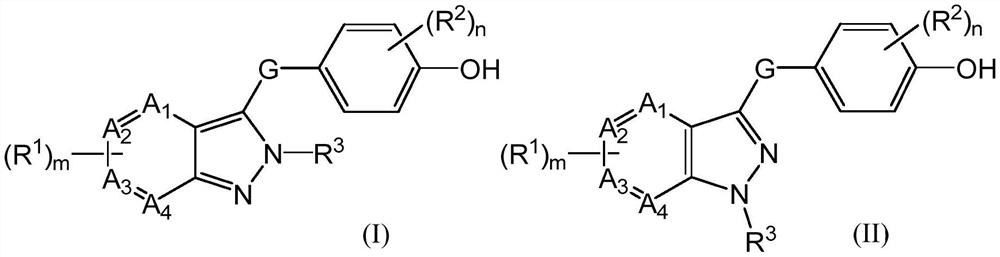
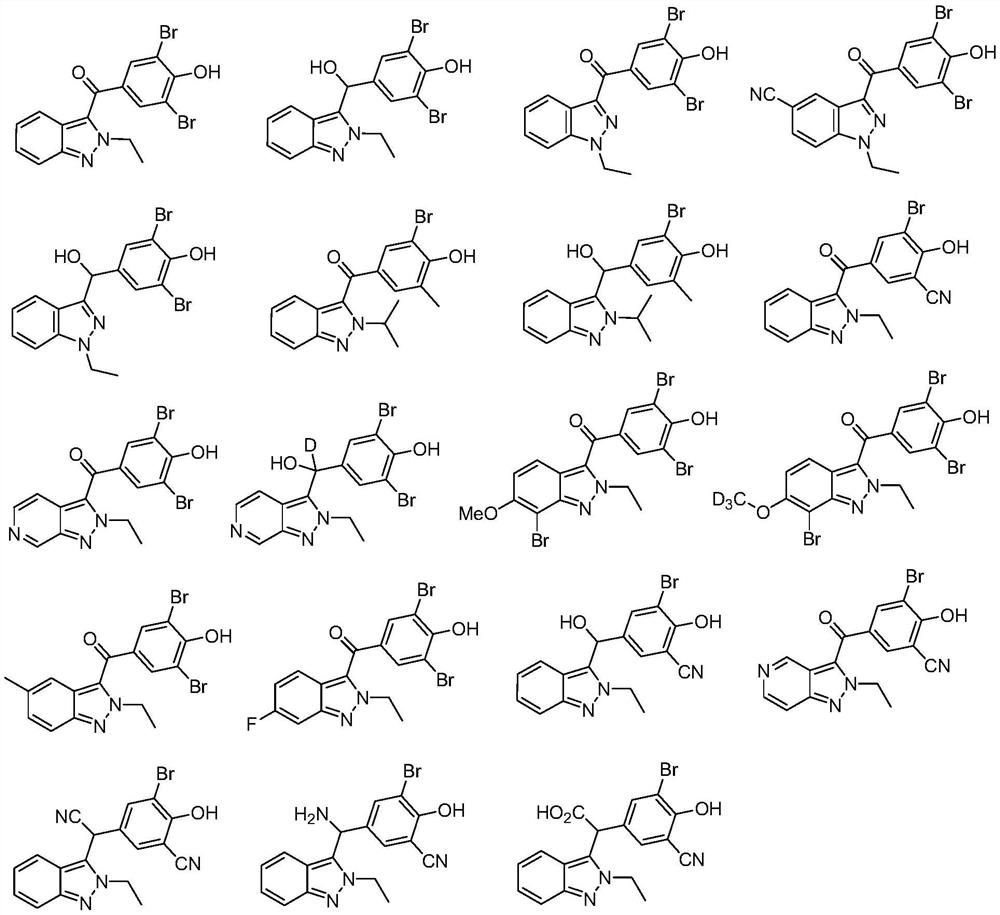
A class of urat1 inhibitors and applications thereof
一种化合物、药学的技术,应用在URAT1抑制剂化合物领域,能够解决严重不良事件发生率高、缺乏抗痛风药物、不良反应等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Synthesis of (3,5-dibromo-4-hydroxyphenyl)(2-ethyl-2H-indazol-3-yl)methanone (6)
[0063]
[0064] Step A: A mixture containing indazole (5.00 g, 42.3 mmol), ethyl iodide (13.2 g, 90.3 mmol), potassium hydroxide (5.50 g, 98.0 mmol) and ethanol (60 mL) was stirred at 65 °C for 5 hours . Cool to room temperature and filter to remove insoluble matter. The solvent was evaporated under reduced pressure, then water (30 mL) was added, extracted with dichloromethane (20 mL×3), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by column chromatography (200-300 mesh silica gel, ethyl acetate:petroleum ether=1:20-1:5 elution) to obtain 2-ethyl-2H-indazole (1) (1.56g) and 1-ethyl-1H-indazole (2) (3.06g). The yields were 25.2% and 49.5%, respectively. Compound 1: 1 H NMR (DMSO-d 6 , 400MHz) δ8.37(s, 1H), 7.69(d, J=8.4Hz, 1H), 7.60(d, J=8.4Hz, 1H), 7.24-7.20(m, 1H), 7.05-7.01(m , 1H), 4.46 ...
Embodiment 2
[0069] Example 2: Synthesis of 2,6-dibromo-4-[(2-ethyl-2H-indazol-3-yl)hydroxymethyl]phenol (7)
[0070]
[0071] Sodium borohydride (90mg, 2.38mmol) was added to a solution of compound 6 (100mg, 0.236mmol) in methanol (10mL). After the addition was complete, the resulting mixture was stirred at reflux for 30 minutes, and then sodium borohydride (90mg, 2.38mmol) was added. ), stirring was continued for 1 hour under reflux. Water (20 mL) was added, the pH value was adjusted to 6-7 with 2M aqueous citric acid solution, extracted with ethyl acetate (20 mL×3), the combined organic phases were washed with water (20 mL), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by column chromatography (200-300 mesh silica gel, ethyl acetate:petroleum ether=1:20-1:3 elution) to obtain 2,6-dibromo-4-[(2 -Ethyl-2H-indazol-3-yl)hydroxymethyl]phenol (7). 1 H NMR (DMSO-d 6 , 400MHz) δ9.99(s, 1H), 7.55(d, J=8.8Hz, 1H), 7.5...
Embodiment 3
[0072] Example 3: Synthesis of (3,5-dibromo-4-hydroxyphenyl)(1-ethyl-1H-indazol-3-yl)methanone (10)
[0073]
[0074] Step A: A mixture containing compound 2 (2.59g, 17.7mmol), p-methoxybenzoyl chloride (3.02g, 17.7mmol) and anhydrous aluminum chloride (3.54g, 26.6mmol) was stirred overnight at 105°C . Cool to room temperature, add water (30 mL) and ethyl acetate (30 mL), and stir for about 5 minutes. The layers were separated, the aqueous layer was extracted with ethyl acetate (10 mL×3), the combined organic phases were washed with water (15 mL), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by column chromatography (200-300 mesh silica gel, ethyl acetate:petroleum ether = 1:25-1:15 elution) to obtain (1-ethyl-1H-indazole-3 -yl)(4-methoxyphenyl)methanone (8) (990 mg). The yield was 21.1%. 1 H NMR (DMSO-d 6 , 400MHz) δ8.34-8.29(m, 3H), 7.88(d, J=8.4Hz, 1H), 7.56-7.52(m, 1H), 7.41-7.37(m, 1H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com