Thermosensitive non-steroidal anti-inflammatory drug solid dispersion and instant tablet and preparation method thereof
A non-steroidal anti-inflammatory drug and solid dispersion technology, which is applied in the direction of medical preparations, anti-inflammatory agents, and pharmaceutical formulations of non-active ingredients, and can solve the problem of poor, slow release of non-steroidal anti-inflammatory drugs. and other issues, to achieve the effect of improved compressibility, strong interaction, increased fluidity and compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis and NMR Characterization of Thermosensitive Excipients
[0048] 1. Synthesis and NMR characterization of poly(N-isopropylacrylamide)
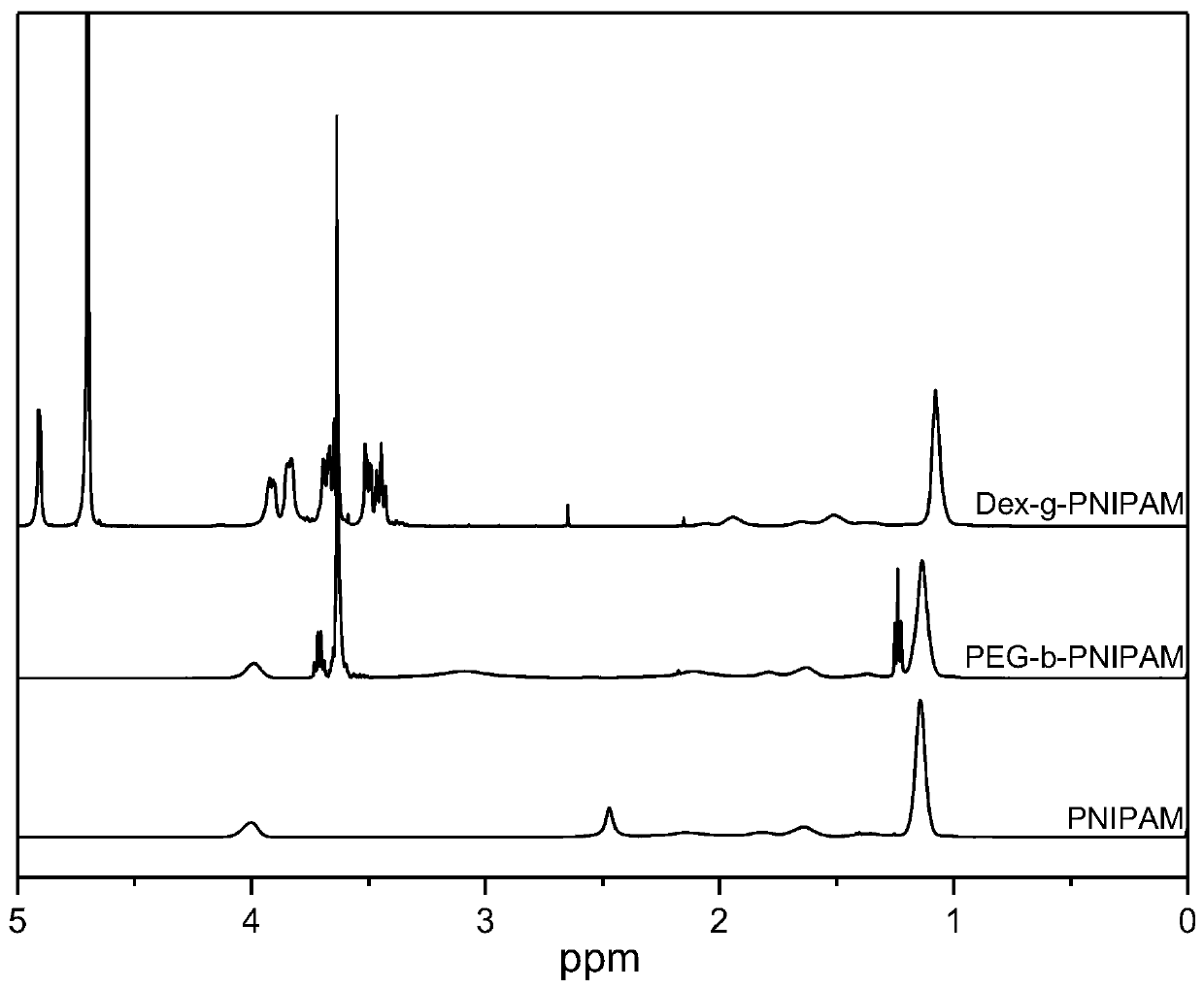
[0049] Dissolve 6 g of N-isopropylacrylamide (NIPAM) crystals in a three-necked bottle filled with 50 mL of deionized water, drum N 2 After 30 minutes, it was heated and stabilized in a constant temperature water bath at 60°C under magnetic stirring, and then injected into 5 mL of aqueous solution containing 30 mg of ammonium persulfate, and reacted for 7 hours. After the reaction was completed, the reaction solution was transferred to a dialysis bag (MWCO: 3500) for dialysis for 3 days, and the product was collected after freeze-drying. Dissolve the sample in heavy water to prepare a solution with a concentration of 20mg / mL, and measure its H NMR spectrum after constant temperature at 25°C for 10min, as shown in figure 1 As shown, it was confirmed that PNIPAM was synthesized successfully.
[0050] 2. Synthesis an...
Embodiment 2
[0054] Example 2: Preparation and structural characterization of thermosensitive ibuprofen solid dispersion
[0055] 1. Preparation and characterization of poly(N-isopropylacrylamide)-ibuprofen solid dispersion
[0056] The PNIPAM-ibuprofen solid dispersion is prepared by an anti-solvent co-precipitation method, specifically a preparation method in which an organic solvent is removed by dialysis. Dissolve 100mg of poly(N-isopropylacrylamide) (PNIPAM) and 91mg or 182mg of ibuprofen in 30mL of dimethyl sulfoxide (DMSO), and transfer to a dialysis bag (MWCO: 3500) after complete dissolution. 1 L of deionized water with a pH of about 3 was dialyzed. Change the water every 3 hours for the first 12 hours, and every 6 hours for the next 36 hours. After the dialysis, the solution in the dialysis bag was centrifuged at 3000 rpm for 10 min, the precipitate was discarded, and the solution was freeze-dried to obtain a PNIPAM-ibuprofen solid dispersion.
[0057] The content of ibuprofen...
Embodiment 3
[0070] Example 3: Research on thermosensitive behavior and in vitro release of ibuprofen solid dispersion
[0071] 1. The average hydrated particle size and intuitive temperature sensitivity of ibuprofen solid dispersion
[0072] Utilize dynamic light scattering to measure the hydration diameter of ibuprofen solid dispersion 3, solid dispersion 4 below its phase transition temperature (room temperature) and above (37 ℃) respectively, as Figure 4As shown, the temperature-sensitive carrier dextran-g-poly(N-isopropylacrylamide) (Dex-g-PNIPAM) has a particle size of about 20nm at room temperature, and when the temperature rises to 37°C, the solution forms a particle size of 100nm micelles. After the drug is entrapped, the system appears as an emulsion at room temperature. After the temperature rises, the particle size decreases from 260nm to about 140nm, and the particle size change is reversible. This shows that when the temperature is lower than the lower critical solution te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com