Spermine-modified pullulan as an immunopotentiator
A technology of pullulan polysaccharide and spermine, which is applied in the field of pullulan polysaccharide, can solve the problem that the application of immune enhancer has not yet been reported publicly, and achieves the effect of good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Refining of pullulan
[0026] PS can be synthesized from commercially obtained pullulan or its further refined products. A typical refining process is briefly described as follows: commercial pullulan (purchased from Tokyo Chemical Industry, with an average molecular weight of about 100,000) is heated and hydrolyzed in an acid solution, and is refined by fractional precipitation by gradually adding ethanol to the hydrolyzed product, thereby A series of pullulan with different molecular weights was obtained. The typical hydrolysis reaction conditions are: hydrolysis in 0.6mol / L sulfuric acid solution at 80°C for 90 minutes, at this time the average molecular weight of the pullulan hydrolyzate is about 50,000. Pullulan with one or more molecular weights (typical molecular weight between 5,000-100,000, especially around 50,000) can be selected for further synthesis of PS.
Embodiment 2
[0027] Embodiment 2: the preparation method of PS
[0028] Coupling agents can be used, such as N,N'-carbonyldiimidazole (N,N'-Carbonyldiimidazole, CDI), N,N'-disuccinimidyl carbonate (N,N'-Disuccinimidyl carbonate, DSC) , Sodium periodate, galactose oxidase enzymes, reactive alkyl halogen compounds, isocyanates, etc., directly or indirectly couple spermine molecules to the hydroxyl groups of pullulan; or open C-C bonds through oxidants such as sodium periodate to form aldehydes Active functional groups such as radicals, so as to further directly or indirectly couple spermine molecules to the carbon skeleton of pullulan.
[0029] Typical reaction conditions are as follows: 3.74 g of spermine and 450 mg of CDI were added to 100 mL of dimethyl sulfoxide (DMSO) containing 100 mg of pullulan, stirred at 35 °C for 20 h, and then the mixture was dialyzed for 2 days, the dialysis molecular weight cut-off was 8-14kD. Freeze-drying is performed after dialysis to obtain PS powder. The...
Embodiment 3
[0030] Embodiment 3: CCK8 method detects the impact of PS on mouse macrophage activity
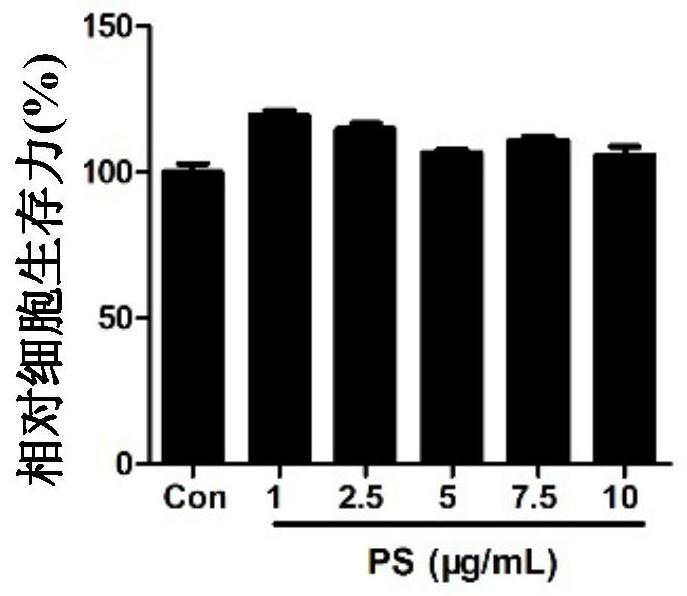
[0031] The mouse macrophage RAW 264.7 was used for the experiment, and the RAW 264.7 cells were planted in a 96-well plate according to 20,000 cells per well. After the cells adhered to the wall and grew well, different concentrations of PS (1, 2.5, 5, 7.5 , 10 μg / mL) were incubated with the cells for 24 hours. After washing twice with PBS, add CCK8 detection reagent (100 μL medium + 10 μL CCK8), put the culture plate in the incubator for 2 hours, absorb the supernatant, detect the absorbance at 450 / 630 nm, and organize the data.
[0032] The result is as figure 1 As shown, there was no significant difference in cell viability compared with the untreated control group, indicating that PS concentrations less than 10 μg / mL did not affect cell viability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com