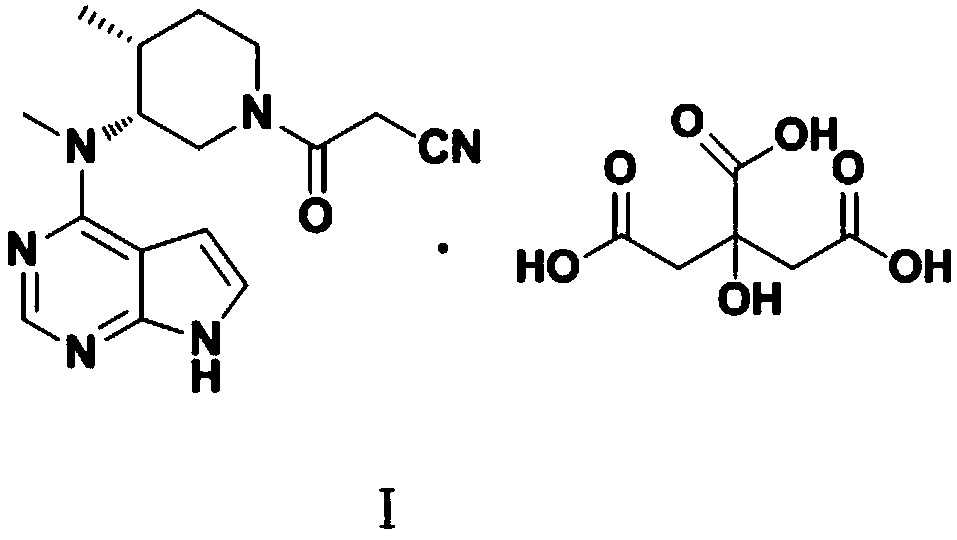
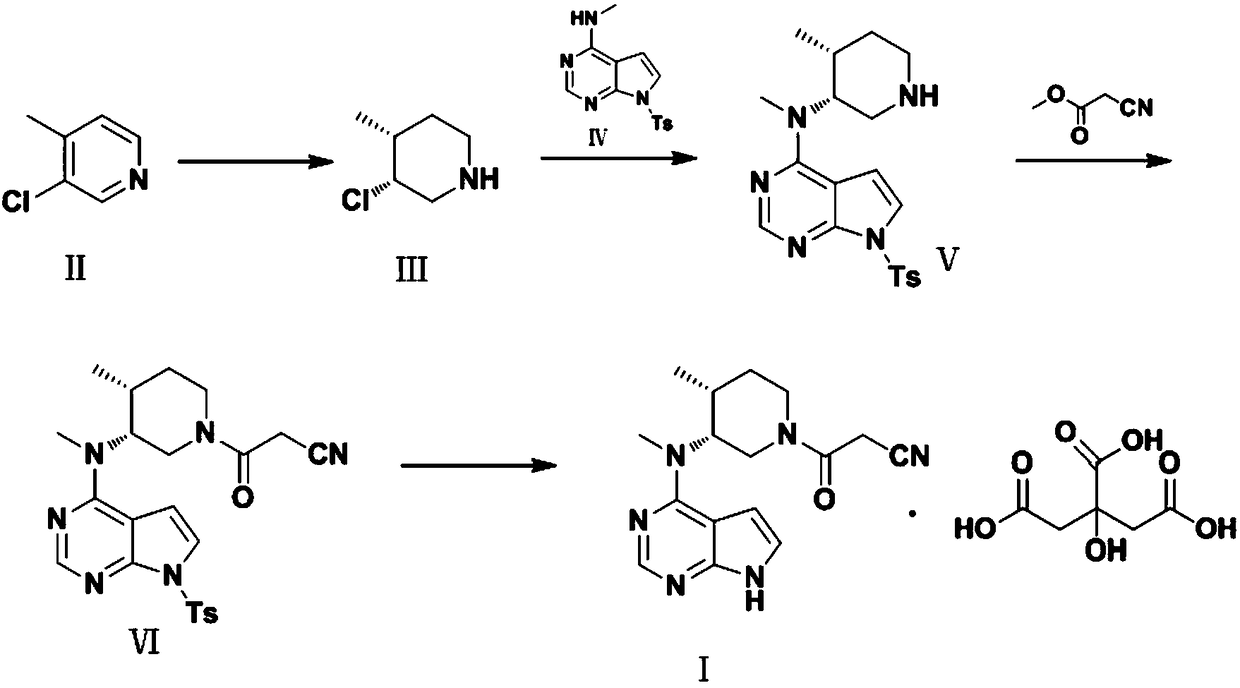
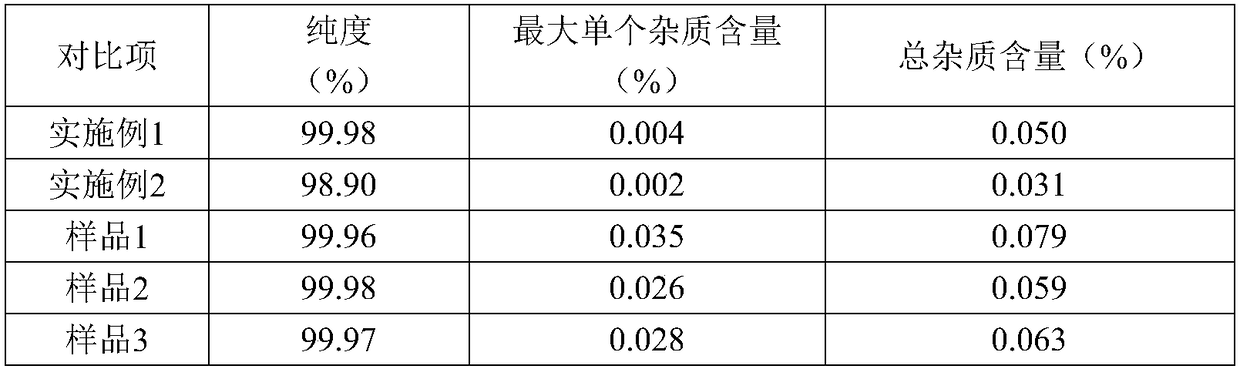
Refining method of tofacitinib citrate
A technology of tofacitinib and purification method, applied in the field of medicine, can solve the problem of high content of maximum single impurity and total impurity, and achieve the effects of easy operation, few by-products and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Preparation of compound Ⅳ
[0029] Add 74.08g (0.5mol) of N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine, 20g of sodium hydroxide, and 1000mL of acetonitrile into the reaction flask, stir at room temperature at 30°C, and then dropwise add 95.35 g (0.5 mol) of methanesulfonyl chloride was added after dropping, and the temperature was raised to 50° C. to react, and the reaction was monitored by TLC. After the reaction is complete, cool to room temperature, remove the solvent by rotary evaporation, add 1000mL of water and 1000mL of ethyl acetate, stir, let stand for liquid separation, extract the water layer with 800mL of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and dry under reduced pressure 146.57g of compound III was obtained, with a yield of 96.8% and a purity of 99.85%.
Embodiment 1
[0031] (1) Synthesis of Tofacitinib Citrate Crude Product
[0032] a. Under nitrogen protection, (0.194mmol) [Ir(COD)Cl]2 (1,5-cyclooctadiene iridium chloride dimer), R-(+)-1 ,1'-binaphthyl-2,2'-diphenylphosphine (0.192mmol), 150ml of toluene, stirred at room temperature for 20min, then added 20.59g of potassium iodide (0.0962mol) and 12.27g of 3-chloro-4-methylpyridine ( 0.0962mol), and put the reaction bottle into a stainless steel autoclave, replace it with hydrogen for three times, and finally rush into the required hydrogen pressure of 600psi. After reacting at room temperature for 12 hours, release the hydrogen slowly, and dilute the reaction system with 150mL of dichloromethane. Add 150mL saturated sodium carbonate solution, stir for 15min, separate the organic layer, then extract the aqueous layer with dichloromethane (3×150mL), combine the organic layers with Na 2 SO 4 After drying, the solvent was removed to obtain 17.89 g of compound III, with a yield of 99.2%, an...
Embodiment 2
[0039] (1) Synthesis of Tofacitinib Citrate Crude Product
[0040]a. Under nitrogen protection, add (0.966mmol) [Ir(COD)Cl] 2 (1,5-cyclooctadiene iridium chloride dimer), R-(+)-1 ,1'-binaphthyl-2,2'-diphenylphosphine (0.193mmol), 150ml of toluene, stirred at room temperature for 20min, then added 20.67g of potassium iodide (0.0966mol) and 12.32g of 3-chloro-4-methylpyridine ( 0.0966mol), and put the reaction bottle into a stainless steel autoclave, replace it with hydrogen for three times, finally rush into the required hydrogen pressure of 600psi, react at room temperature for 12 hours, release hydrogen slowly, and dilute the reaction system with 150mL dichloromethane, Add 150mL saturated sodium carbonate solution, stir for 15min, separate the organic layer, then extract the aqueous layer with dichloromethane (3×150mL), combine the organic layers with Na 2 SO 4 After drying, the solvent was removed to obtain 17.73 g of compound III, with a yield of 95.8%, an HPLC purity of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com