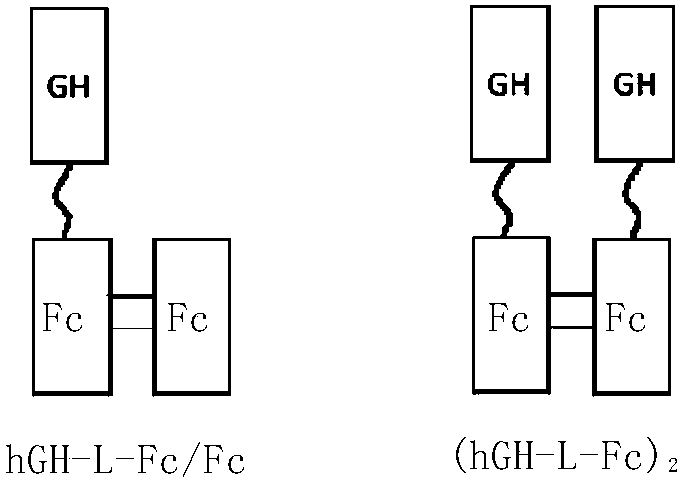
Recombinant long-acting HGH (human growth hormone) fusion protein and preparation and application thereof
A fusion protein and growth hormone technology, applied in the direction of growth hormone, animal/human protein, hormone peptide, etc., can solve the problems of low long-term effect, loss of activity, and lower specific activity of growth hormone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0139] Preparation of fusion protein
[0140] As used herein, "isolated" means that the material is separated from its original environment (if the material is native, the original environment is the natural environment). For example, polynucleotides and polypeptides in the natural state in living cells are not isolated and purified, but the same polynucleotides or polypeptides are isolated and purified if they are separated from other substances that exist together in the natural state.
[0141] As used herein, "isolated recombinant fusion protein" means that the recombinant fusion protein is substantially free of other proteins, lipids, carbohydrates or other substances with which it is naturally associated. Those skilled in the art can purify recombinant fusion proteins using standard protein purification techniques. Essentially pure proteins yield a single major band on non-reducing polyacrylamide gels.
[0142] A polynucleotide of the invention may be in the form of DNA...
Embodiment 1
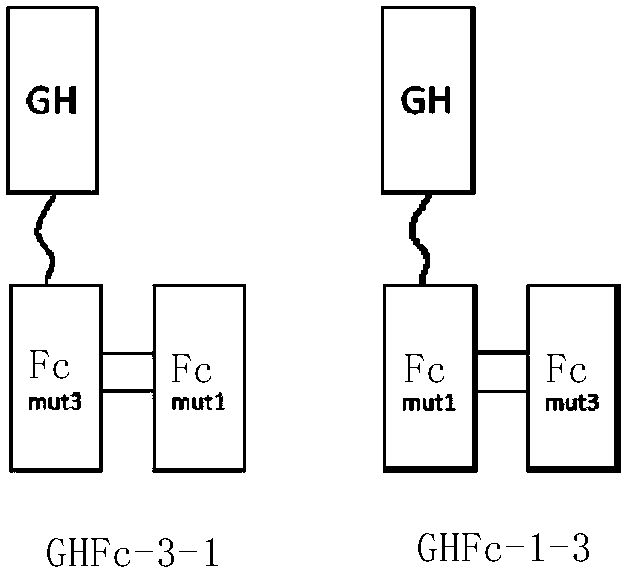
[0187] Embodiment 1 investigates the proportion of monomeric molecules in cell culture of GHFc-1-3 and GHFc-3-1 two kinds of molecules
[0188] In order to make it easier for GHFc molecules to form a monomeric form in the cell, that is, the GH-(Fc)*2 form, the present invention mutates some amino acids of the IgG4-Fc part, thereby making it easier to form the GH-(Fc)*2 form. And the connection between Fc and GH of different mutations is also the object of investigation in the present invention, and two kinds of molecules have been designed (see figure 2 ):
[0189] One of the molecules is a GH sequence using a linker peptide and IgG4-Fc containing (1), (2), (3), (4) in the first type of mutation and (1) in the above-mentioned second type of mutation (i.e. M2-IgG4-Fc, referred to as mut3) is linked to IgG4-Fc containing (1), (2), (3), (4) in the above-mentioned first type of mutation and (2) in the above-mentioned second type of mutation (ie M3-IgG4-Fc, referred to as mut 1)...
Embodiment 2
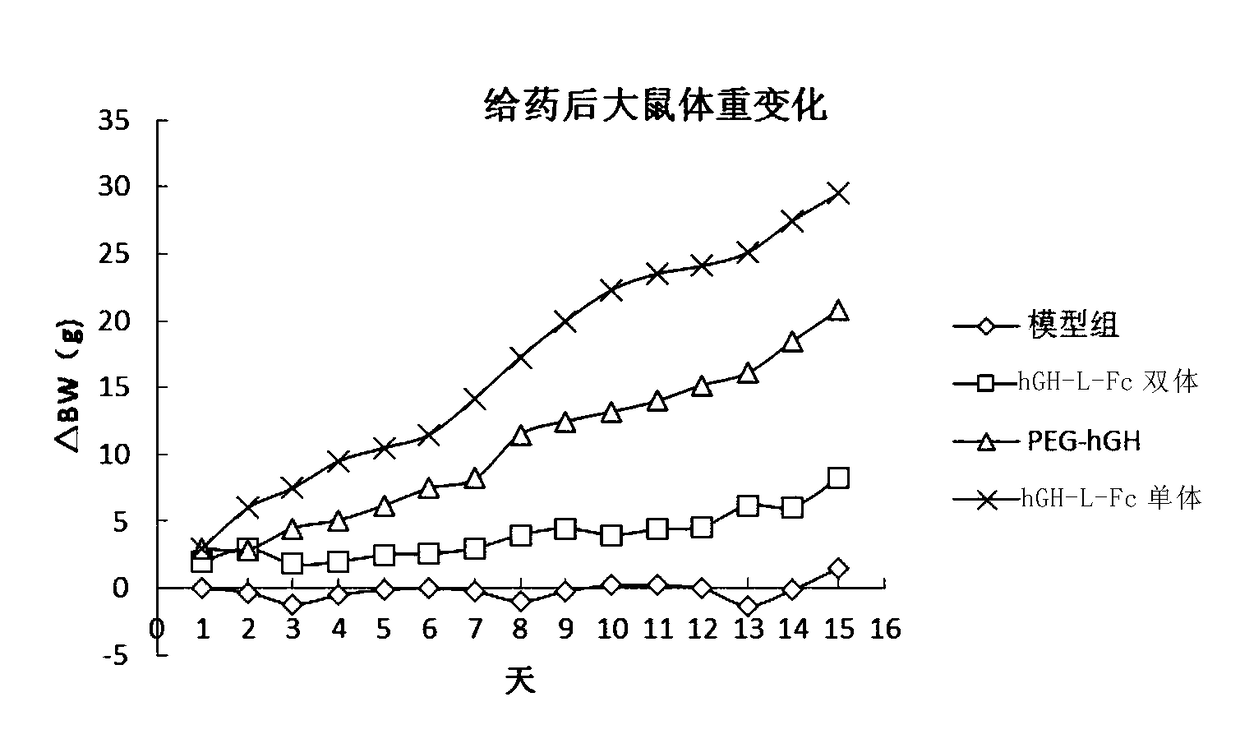
[0203] Example 2 In vitro biological activity analysis of hGH-L-Fc
[0204] The growth hormone biological activity assay is a cell-based receptor titer increase assay, namely the BAF method.
[0205] The BAF cell line responds to GH in a dose-dependent growth pattern, so it can be used to evaluate the role of GH in proliferation assays. The BAF cell line was cultured in DMEM high-glucose medium supplemented with 10% horse serum for 16 hours. , adjust the cell density to 3*10^5 / ml, take 100 microliters and drop them into 96-well plates after mixing. Dilute the standard GH solution to a final concentration of 0.01-1000.00ug / L, add 10ul of the standard GH solution dropwise to a 96-well plate, and incubate for 22 hours. Add 22ul of reaction solution to each well, continue to incubate for 3 hours, and measure the absorbance at 490nm. Draw the standard curve of the relationship between absorbance value and GH biological activity equivalent. 10ul of different growth hormone compou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com