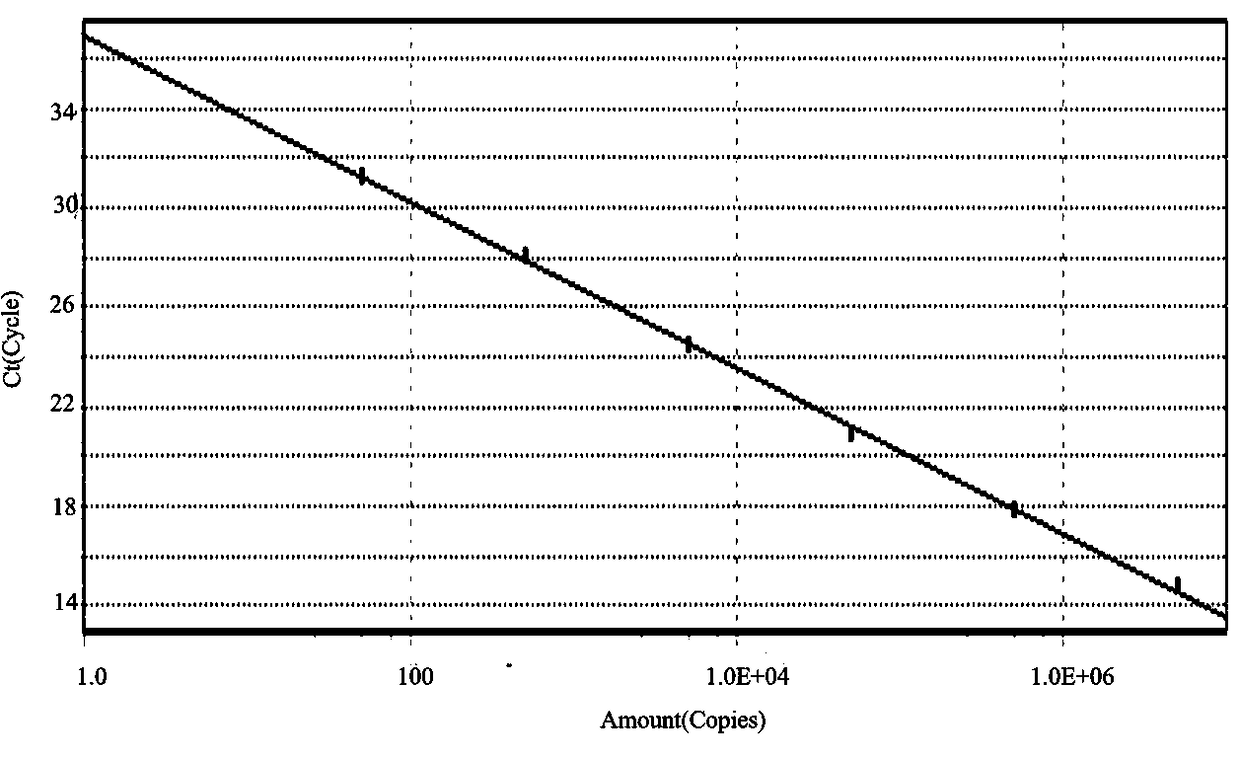
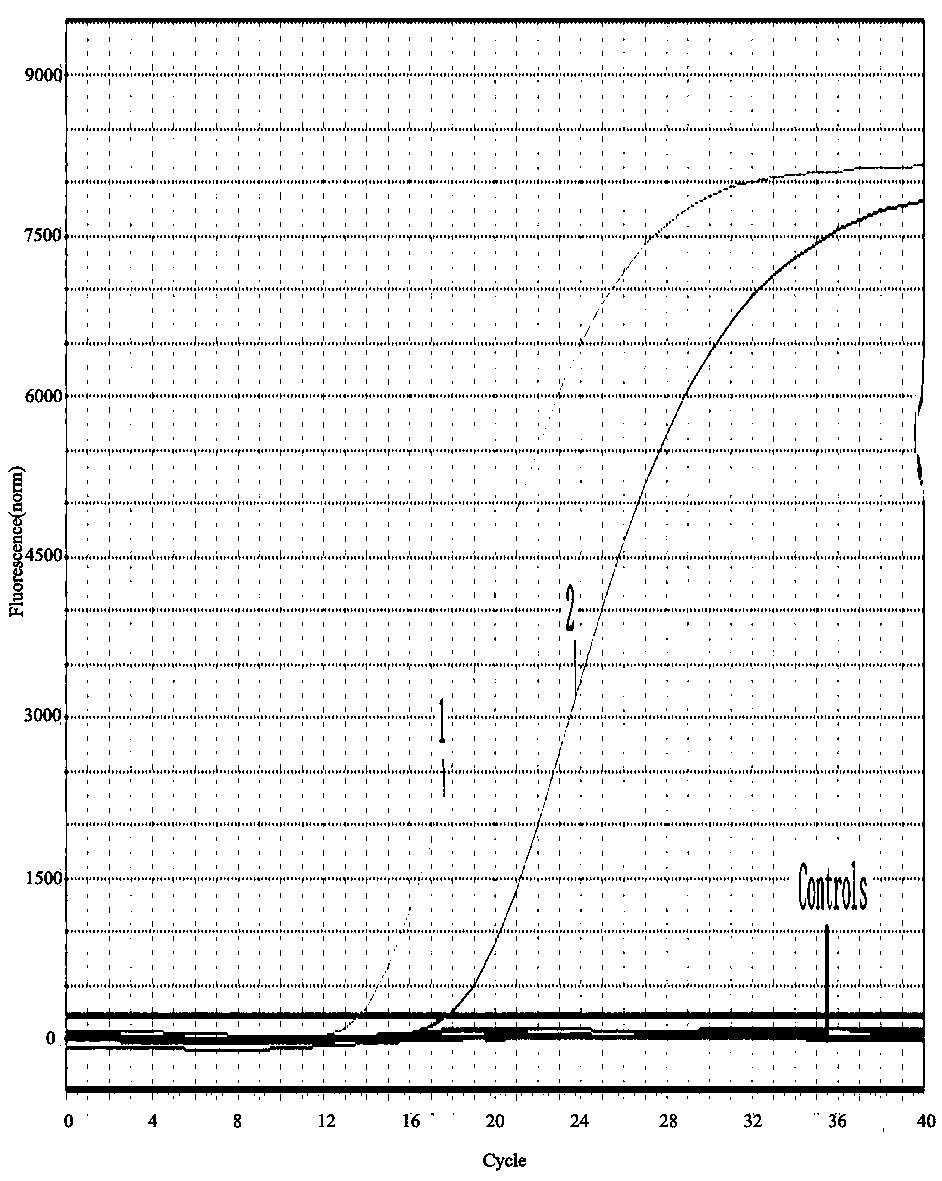
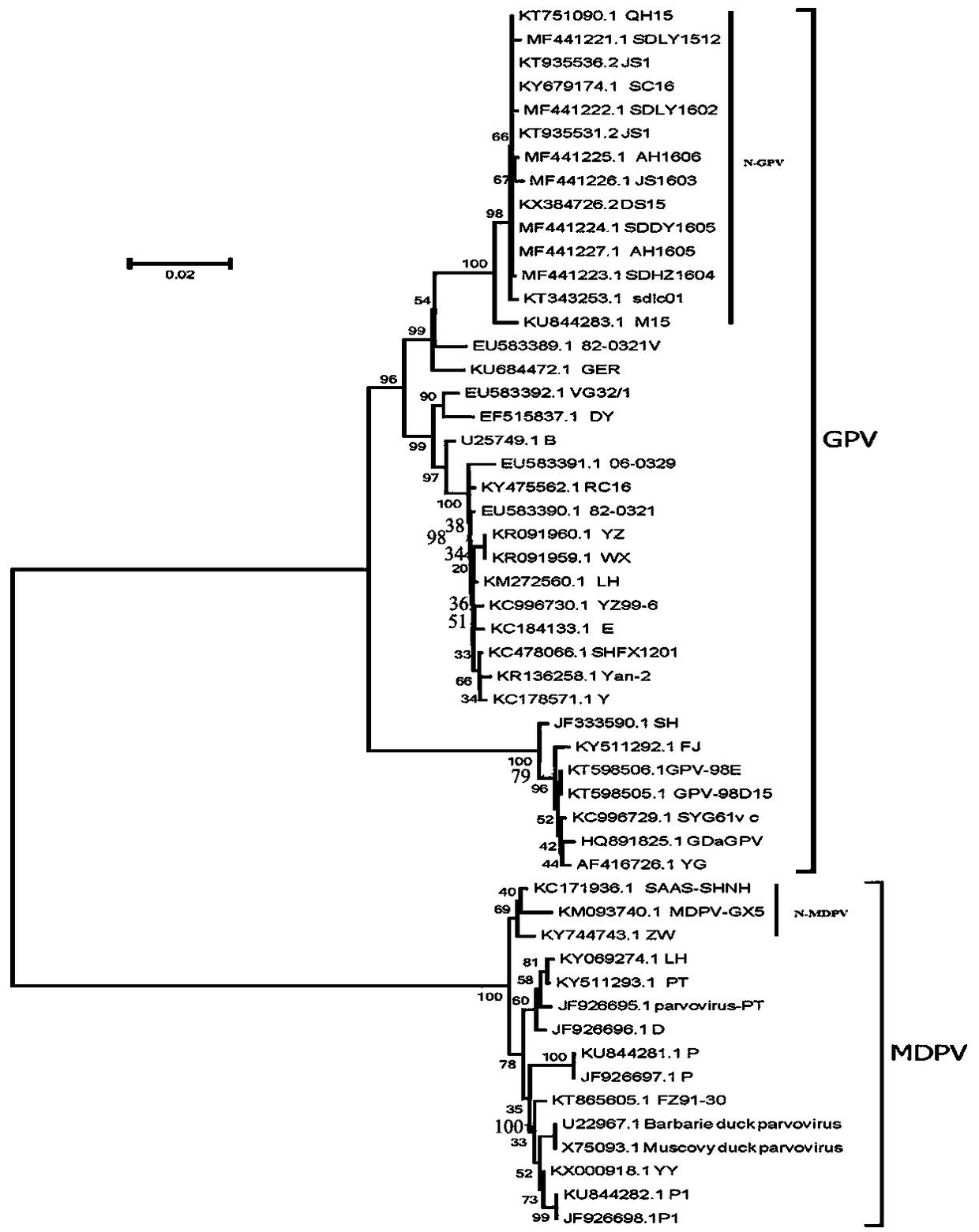
GPV and N-GPV common type detection primer and probe
A detection primer and a general-purpose technology, applied in the field of avian disease, can solve problems such as erroneous results, affecting the accurate diagnosis and treatment of epidemic diseases, and achieve the effects of rapid detection, high sensitivity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Related test pathogens
[0029] 1.1 Test strains
[0030] GPV and N-GPV were separated, identified and preserved by the Institute of Animal Husbandry and Veterinary Medicine, Fujian Academy of Agricultural Sciences.
[0031] 1.2 Test control strains and strains
[0032] Common nucleic acid types in mule ducks are pathogens of DNA, such as MDPV, N-MDPV, duck adenovirus type A (DAdV-A), duck plague virus (DEV), duck Escherichia coli (E. coli), duck plague Richter Bacillus (RA) and Pasteurella multocida (PM) from ducks were identified and preserved by the Institute of Animal Husbandry and Veterinary Medicine, Fujian Academy of Agricultural Sciences.
[0033] , Primer and probe design and analysis
[0034] 2.1 Comparative analysis of NS genes
[0035] The NS gene sequences of 52 waterfowl parvoviruses uploaded in GenBank [including 37 GPV (including 14 N-GPV) and 15 MDPV] NS gene sequences were compared for nucleotide homology. The results show that the nucleotide homology between...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| PCR efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com