Method of recycling butene by alkyne selective hydrogenation
A technology for selective hydrogenation and alkyne, applied in chemical recovery, chemical instruments and methods, hydrogenation to hydrocarbons, etc., which can solve the problems of easy agglomeration, poisoning and deactivation of active components
- Summary
- Abstract
- Description
- Claims
- Application Information
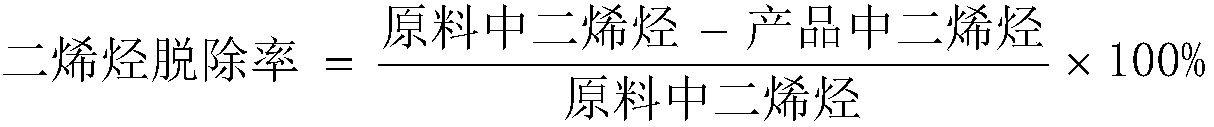
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Embodiment uses the preparation of catalyst 1~6
[0055] Preparation of Catalyst C1:
[0056] Concentrate 4L to 50g Al 2 o 3 / L of sodium metaaluminate solution is placed in a stainless steel container with a stirrer and gas can be introduced into the bottom of the tank, the nickel nitrate solution is prepared and placed in a high-position container, a mixed gas of carbon dioxide and air is introduced, and the peristaltic pump is started at the same time Control the flow rate and drop the prepared nickel nitrate solution, the carbon dioxide concentration in the mixed gas is 60v%, and the flow rate is 3Nm 3 / h, the reaction temperature is 30°C, the pH value at the end of the reaction is 10.0, stop feeding carbon dioxide, age for 30 minutes, filter and separate the mother liquor, wash, and dry at 120°C for 5 hours to obtain pseudo-boehmite containing nickel.
[0057] Mix and knead nickel-containing pseudo-boehmite with nitric acid and water, extrude into strips, dry at...
Embodiment 1
[0084] The C4 fraction rich in alkynes is diluted with the C4 raffinate, and the weight ratio of the C4 fraction rich in alkynes to the C4 raffinate is 1:20. The adiabatic reactor adopts a single-stage adiabatic fixed bed, using catalyst C1, and the catalyst is reduced at 120°C for 6 hours under a hydrogen atmosphere. The reaction inlet temperature is 40°C, the reaction pressure is 2.0MPa, and the liquid space velocity is 15h -1 , the molar ratio of hydrogen to alkynes+dienes is 5.0, Table 2 is the composition of the materials before and after the reaction.
[0085] Table 2 Composition of materials before and after reaction
[0086]
Embodiment 2
[0088] The C4 fraction rich in alkynes is diluted with C4 after ether, and the weight ratio of the C4 fraction rich in alkynes to C4 after ether is 1:18. The adiabatic reactor adopts a single-stage adiabatic fixed bed, adopts catalyst C2, and the catalyst is reduced at 120°C for 6 hours under a hydrogen atmosphere. The reaction inlet temperature is 38°C, the reaction pressure is 1.5MPa, and the liquid space velocity is 17h -1 , the molar ratio of hydrogen to alkynes+dienes is 4.0, Table 3 is the composition of the materials before and after the reaction.
[0089] Table 3 Composition of materials before and after reaction
[0090]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com