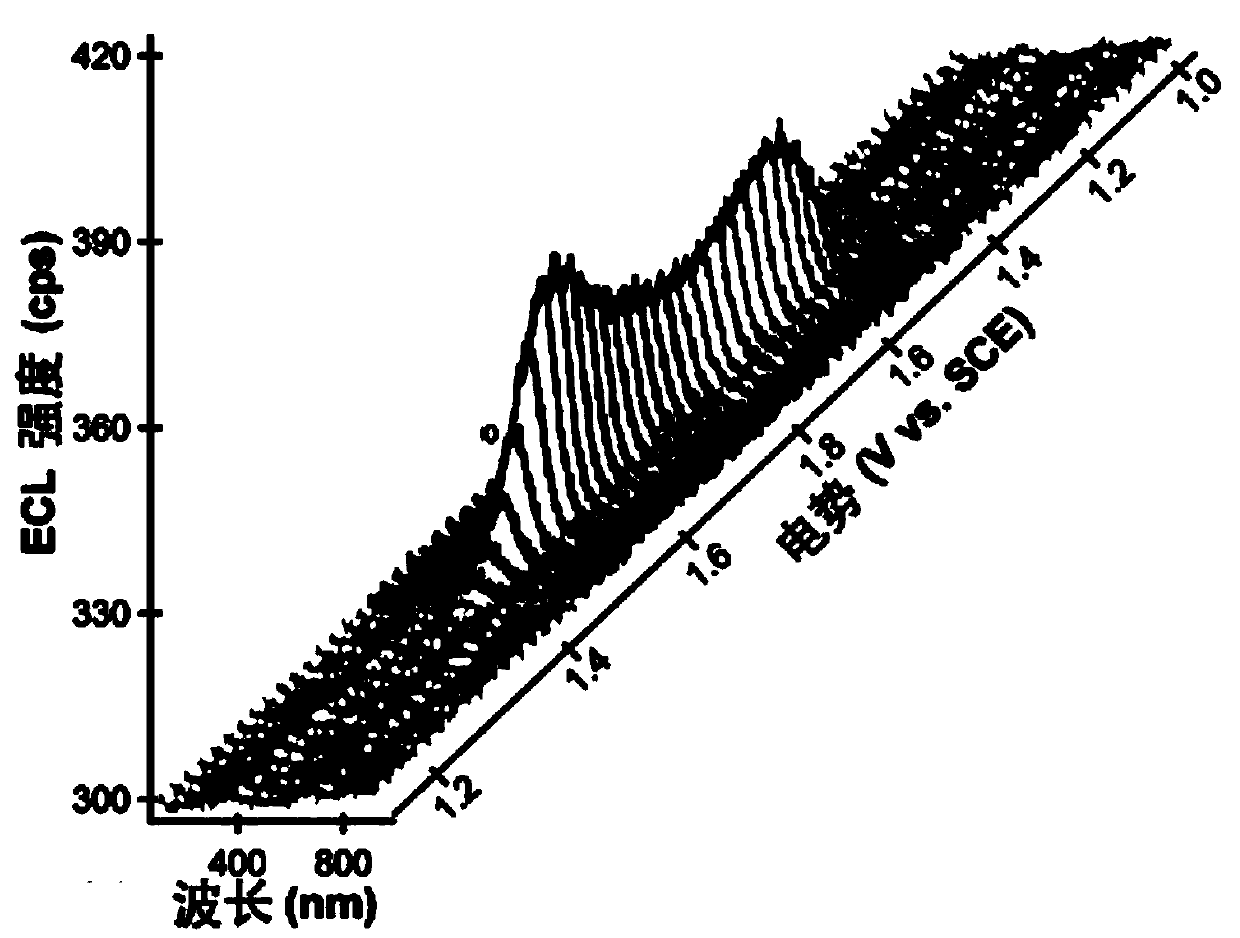
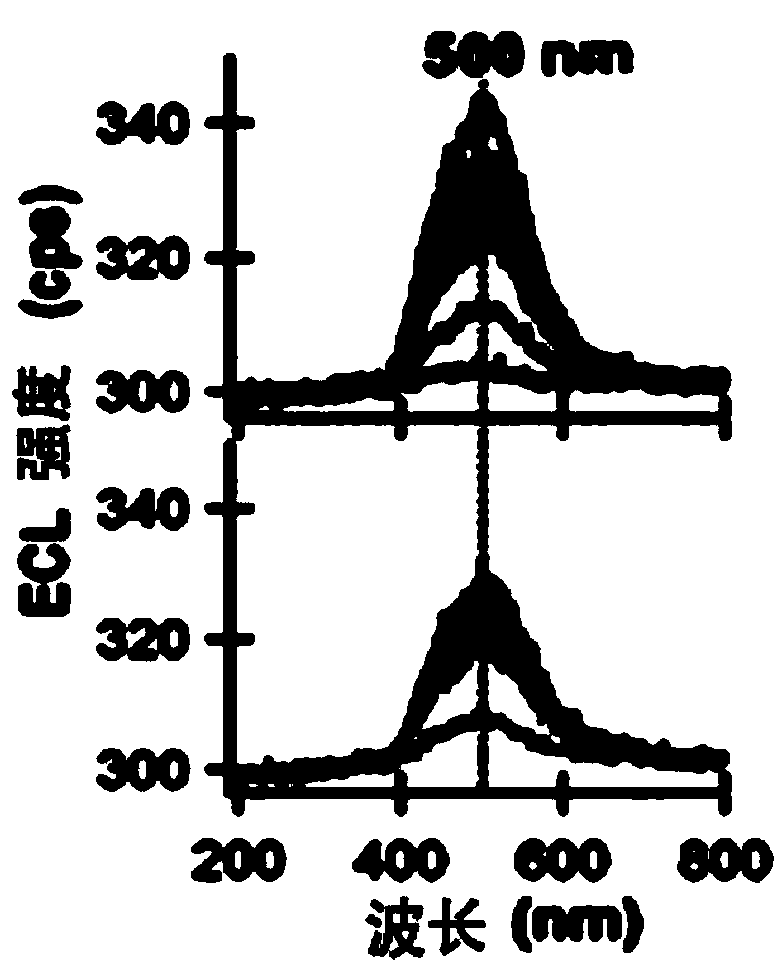
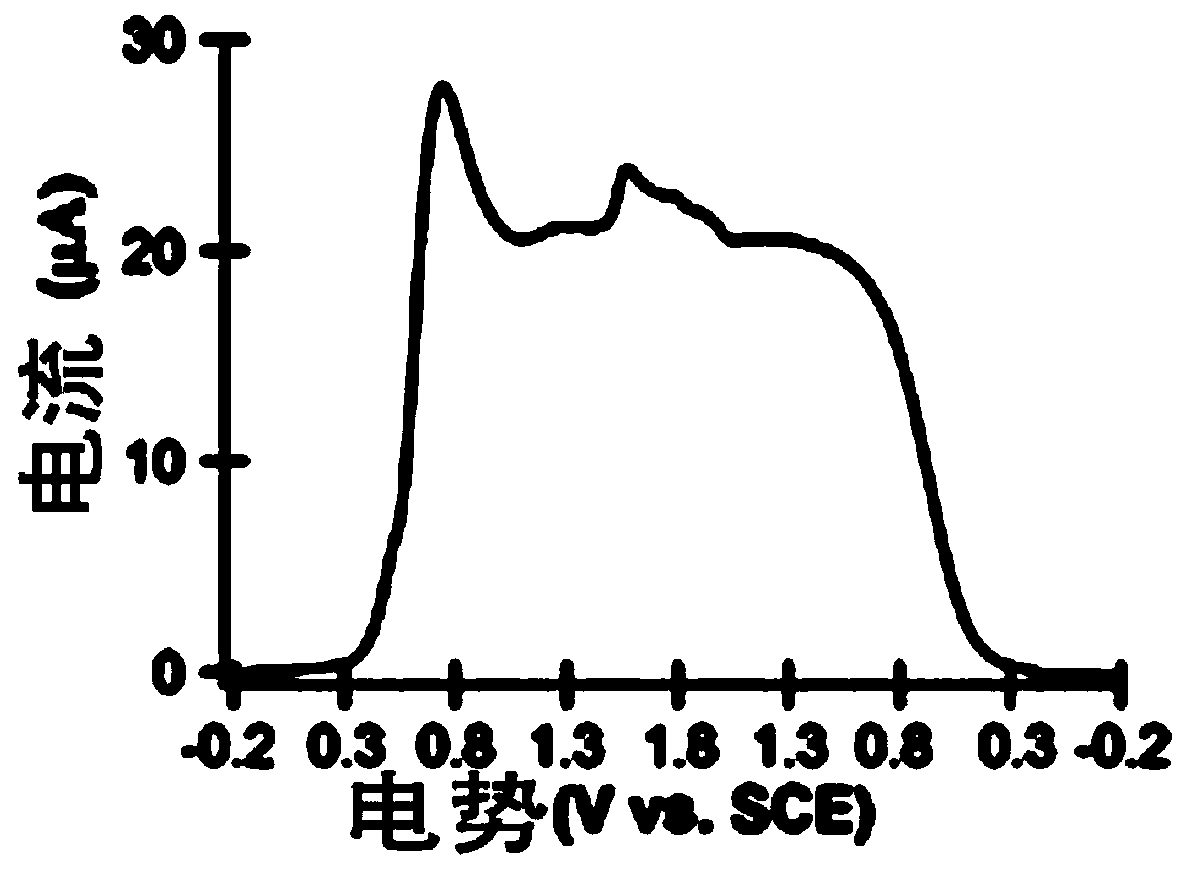
Application of a compound as an electrochemiluminescent material
A technology of luminescent materials and compounds, applied in luminescent materials, organic chemistry, electrical excitation analysis, etc., can solve the problems of high price and low toxicity, and achieve the effects of reducing the use of organic solvents, reducing synthesis costs, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040]In a 250mL round bottom flask, first add 0.5-0.1e.q. of L-proline (preferably 230mg, 0.2mmol) and 1.0-1.5e.q. of malononitrile (preferably 793mg, 12mmol) to dissolve completely, then add 1.0e.q. Benzaldehyde (preferably 1.06g, 10mmol), 1.0~1.2e.q. of 2-amino-5-picoline (preferably 1.296g, 12mmol), placed on an oil bath at 90°C for reaction, TLC (thin layer chromatography ) to complete the reaction for about 8 hours. Post-treatment purification: adding dichloromethane (20mL×3) for extraction, washing with saturated NaCl solution (20mL×1), separating and combining the organic phase and adding anhydrous sodium sulfate for drying. Remove the solvent, separate and purify with silica gel column chromatography, the range of V (petroleum ether): V (ethyl acetate) is 10:1 to 1:1, preferably, V (petroleum ether): V (ethyl acetate) = 3 :1, to obtain pure yellow powder.
[0041] Compound I-1 is tested by proton nuclear magnetic spectrum:
[0042] 1 H NMR (400MHz, CDC...
Embodiment 2
[0044]
[0045] In a 50mL round bottom flask, add compound I-1 (970mg, 3.73mmol) and 15mL of acetic anhydride, heat to 150°C for reaction, TLC (thin layer chromatography) detects that the reaction is complete for about 3.5h. Carry out post-processing purification: remove acetic anhydride, separate and purify with silica gel column chromatography, the scope of V (petroleum ether): V (ethyl acetate) is 1:1 to 1:10, preferred [V (petroleum ether) in the present embodiment ): V (ethyl acetate) = 1:10] to obtain a yellow powder.
[0046] The proton nuclear magnetic spectrum of compound 1-2 is as follows:
[0047] 1 H NMR (400MHz, CDCl 3 )δ9.09(s,1H),7.99(d,J=7.1Hz,2H),7.85(dd,J=8.9,1.6Hz,1H),7.73(d,J=8.9Hz,1H),7.60- 7.42(m,3H),7.26(s,1H),2.47(d,J=35.9Hz,6H).
Embodiment 3
[0049]
[0050] In a 250mL round bottom flask, first add 0.05-0.3e.q. of L-proline (preferably 230.6mg, 2.0mmol) and 1.0-1.5e.q. of malononitrile (preferably 7-93.2mg, 12mmol) to dissolve completely, then add 1.0e.q of 4-bromo-benzaldehyde (1.85g, 10mmol), 1.0~1.5e.q. of 2-amino-5-picoline (preferably 1.296g, 12mmol), placed on a 90°C oil bath for reaction, TLC ( Thin-layer chromatography) detects that the reaction is complete for about 8 hours. Post-treatment purification: adding dichloromethane (20mL×3) for extraction, washing with saturated NaCl solution (20mL×1), separating and combining the organic phase and adding anhydrous sodium sulfate for drying. Remove the solvent, separate and purify with silica gel column chromatography, the range of V (dichloromethane): V (ethyl acetate) is 100:1 to 5:1, preferably, V (dichloromethane): V (ethyl acetate) =30:1, a yellow powder was obtained. The yield of this reaction reaches 56%.
[0051] Compound II-1 is tested by H NMR sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com