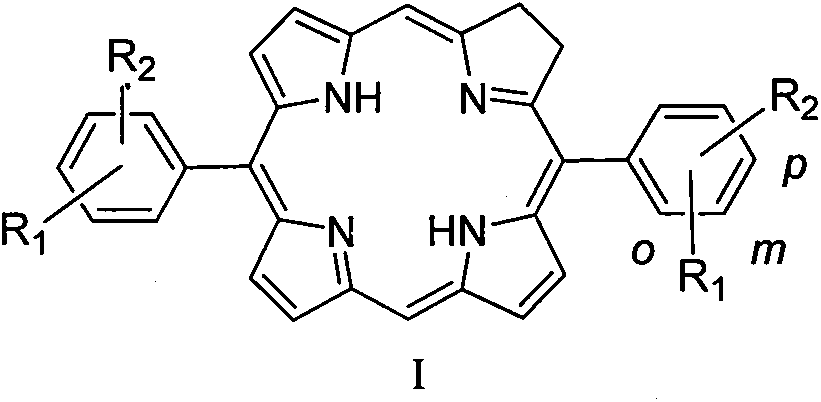
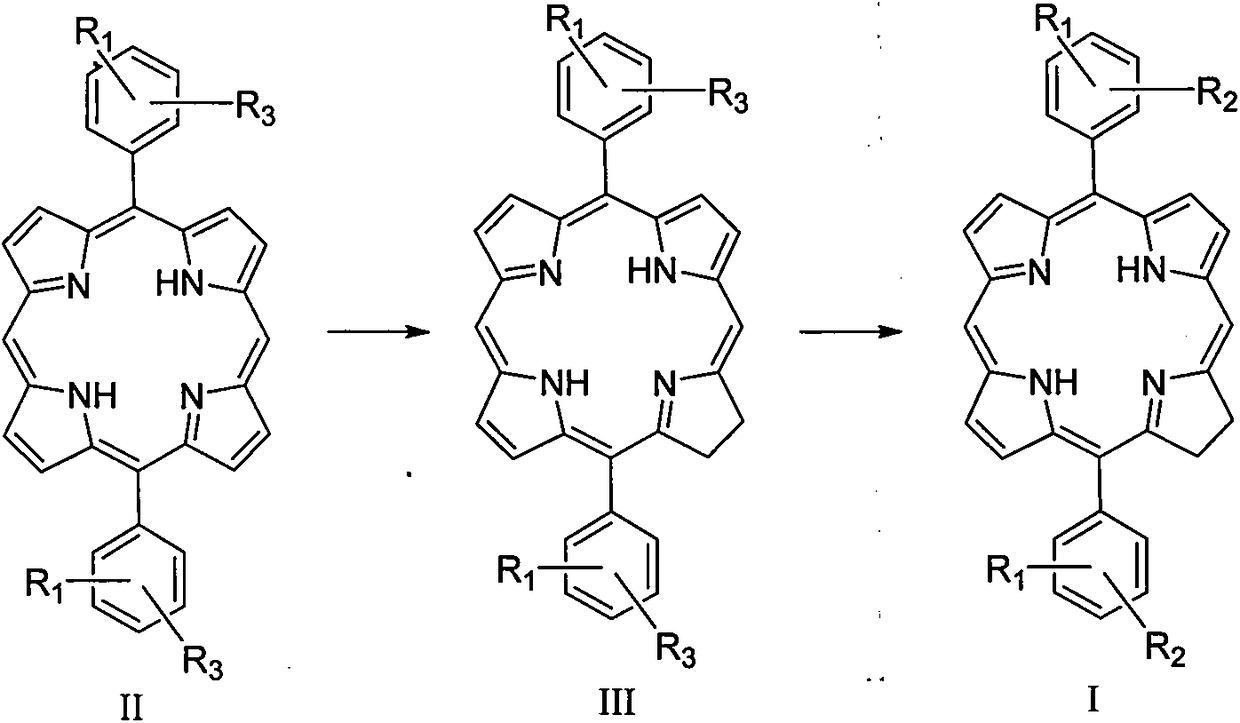
Diphenyldihydroporphin compound, preparation method and application thereof
A diphenyl chlorin compound technology, applied in the field of diphenyl chlorin compound and its preparation, can solve the problems of skin phototoxicity, unstable structure, complex composition, etc., to reduce phototoxicity Side effects, enhanced photodynamic effects, increased absorption and distribution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
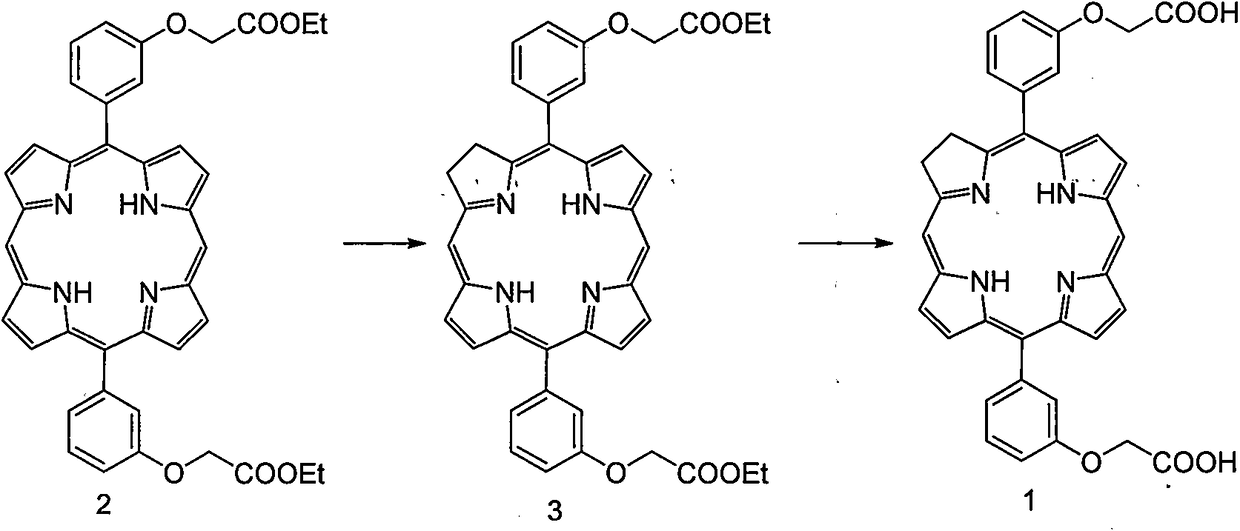
[0027] 5,15-two [(3-carboxymethoxy) phenyl] chlorin (1) synthetic preparation method
[0028]
[0029] Compound 2 (333mg, 0.5mmol) and potassium carbonate (828mg, 6mmol) were added to pyridine (23mL), stirred, and heated to reflux under nitrogen protection; a pyridine solution of p-toluenesulfonyl hydrazide (0.5mol / L) was added dropwise , Thin-layer chromatography (TLC) monitored until the reaction was complete. The reaction solution was cooled, ethyl acetate (100 mL) and distilled water (50 mL) were added, and heated to reflux for 1 h. Cool, neutralize with hydrochloric acid solution (2mol / L), and let stand to separate layers. The organic phase was washed with saturated brine (50 mL×3), dried over anhydrous sodium sulfate, and filtered with suction. The filtrate was stirred at normal temperature, and chlorobenzoquinone was added in batches until the characteristic absorption peak in the ultraviolet-visible spectrum of diphenyltetrahydroporphin compound disappeared; ), 2...
Embodiment 2
[0032] The preparation method of 5,15-bis[(3-carboxypropoxy)phenyl]chlorin (4)
[0033]
[0034] Compound 5 (361mg, 0.5mmol) and potassium carbonate (828mg, 6mmol) were added to pyridine (23mL), stirred, and heated to reflux under nitrogen protection; a pyridine solution of p-toluenesulfonylhydrazide (0.5mol / L) was added dropwise , TLC monitored until the reaction was complete. The reaction solution was cooled, ethyl acetate (100 mL) and distilled water (50 mL) were added, and heated to reflux for 1 h. Cool, neutralize with hydrochloric acid solution (2mol / L), and let stand to separate layers. The organic phase was washed with saturated brine (50 mL×3), dried over anhydrous sodium sulfate, and filtered with suction. The filtrate was stirred at normal temperature, and chlorobenzoquinone was added in batches until the characteristic absorption peak in the ultraviolet-visible spectrum of diphenyltetrahydroporphin compound disappeared; ), 2M sodium hydroxide solution (50mL×3...
Embodiment 3
[0037] The preparation method of 5,15-bis[(3-carboxymethoxy-4-methoxy)phenyl]chlorin (7)
[0038]
[0039] Compound 8 (363mg, 0.5mmol) and potassium carbonate (828mg, 6mmol) were added to pyridine (23mL), stirred, and heated to reflux under nitrogen protection; a pyridine solution of p-toluenesulfonyl hydrazide (0.5mol / L) was added dropwise , TLC monitored until the reaction was complete. The reaction solution was cooled, ethyl acetate (100 mL) and distilled water (50 mL) were added, and heated to reflux for 1 h. Cool, neutralize with hydrochloric acid solution (2mol / L), and let stand to separate layers. The organic phase was washed with saturated brine (50 mL×3), dried over anhydrous sodium sulfate, and filtered with suction. The filtrate was stirred at normal temperature, and chlorobenzoquinone was added in batches until the characteristic absorption peak in the ultraviolet-visible spectrum of diphenyltetrahydroporphin compound disappeared; ), 2M sodium hydroxide solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com