Preparing method for oxide dispersion strengthening iron-cobalt-nickel medium-entropy alloy
A technology of dispersion strengthening and iron-cobalt-nickel, which is applied in the field of medium entropy alloy material preparation, can solve the problems of unsuitability for large-scale production, waste of materials, low powder yield, etc., and achieve suitable for large-scale industrial production, simple preparation process and low cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
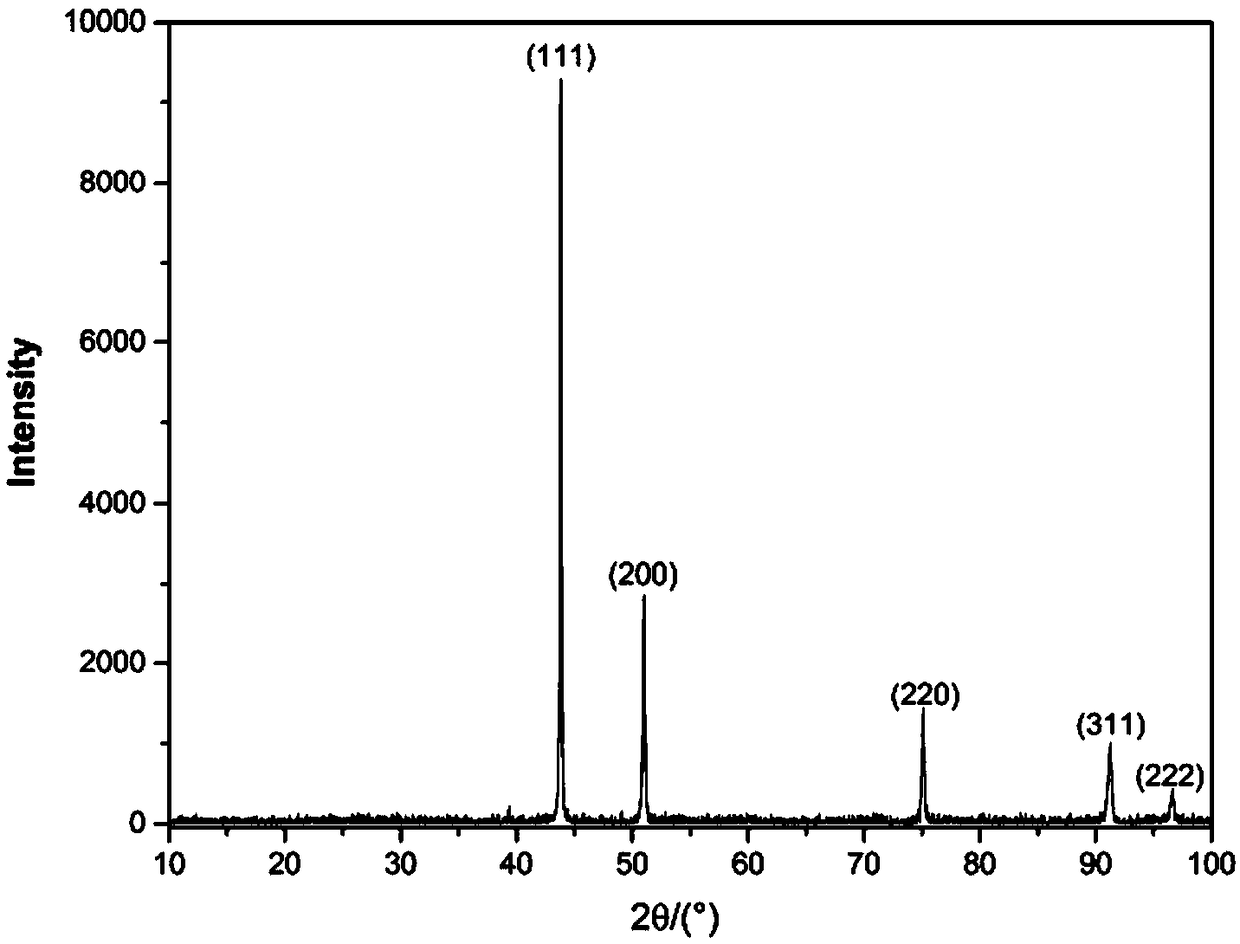
Image
Examples
Embodiment 1
[0027] 1. Weigh 269.38g FeCl_3∙6H 2 O, 237.46 g CoCl 2 ∙6H 2 O, 236.50g NiCl 2 ∙6H 2 O and 1.172g Y (NO 3 ) 3 ∙6H 2 O. These compounds were then dissolved in deionized water to form a dilute solution, and water bath heating and magnetic stirring were carried out during the dissolution process to speed up the dissolution rate.
[0028] 2. Prepare a sodium hydroxide solution with a concentration of 10g / 100ml, and titrate it into the mixed solution prepared in step (1) at a uniform speed. During the titration, the solution is continuously stirred and heated in a water bath at 60°C to make the ferric hydroxide , cobalt hydroxide, nickel hydroxide and yttrium hydroxide are precipitated together, and finally a precipitate in which these hydroxides are evenly mixed is obtained.
[0029] 3. The obtained mixed precipitate was repeatedly suction-filtered and dissolved 5 times, and then washed, and the washed precipitate was put into a vacuum drying oven for drying at 80° C. for ...
Embodiment 2
[0034] 1. Weigh 269.38g FeCl 3 ∙6H 2 O, 237.46 g CoCl 2 ∙6H 2 O, 236.50gNiCl 2 ∙6H 2 O and 2.93g Y(NO 3 ) 3 ∙6H 2 O. These compounds were then dissolved in deionized water to form a dilute solution, and water bath heating and magnetic stirring were carried out during the dissolution process to speed up the dissolution rate.
[0035] 2. Prepare a sodium hydroxide solution with a concentration of 8g / 100ml, and titrate it into the mixed solution prepared in step (1) at a uniform speed. During the titration process, the solution is continuously stirred and heated at 50°C to make ferric hydroxide, Cobalt hydroxide, nickel hydroxide and yttrium hydroxide are precipitated together, and finally a precipitate is obtained in which these hydroxides are uniformly mixed.
[0036] 3. The obtained mixed precipitate was repeatedly suction-filtered and dissolved 5 times, and then washed, and the washed precipitate was put into a vacuum drying oven for drying at 80° C. for 1.5 hours. ...
Embodiment 3
[0040]1. Weigh 269.38g FeCl 3 ∙6H 2 O, 237.46 g CoCl 2 ∙6H 2 O, 236.50gNiCl 2 ∙6H 2 O and 4.102g Y (NO 3 ) 3 ∙6H 2 O. These compounds were then dissolved in deionized water to form a dilute solution, and water bath heating and magnetic stirring were carried out during the dissolution process to speed up the dissolution rate.
[0041] 2. Prepare a sodium hydroxide solution with a concentration of 12g / 100ml, and titrate it into the mixed solution prepared in step (1) at a uniform speed. During the titration process, the solution is continuously stirred and heated at 50°C to make ferric hydroxide, Cobalt hydroxide, nickel hydroxide and yttrium hydroxide are precipitated together, and finally a precipitate is obtained in which these hydroxides are uniformly mixed.
[0042] 3. The obtained mixed precipitate was repeatedly suction-filtered and dissolved 6 times, and then washed, and the washed precipitate was put into a vacuum drying oven for drying at 80° C. for 2 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com