Polysaccharide protein conjugate vaccine and identification method thereof
A polysaccharide protein and conjugated vaccine technology, applied in the medical field, can solve the problems of affecting the test effect, difficult to achieve the antigen concentration of the immune double diffusion method, and unable to produce a precipitation line, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
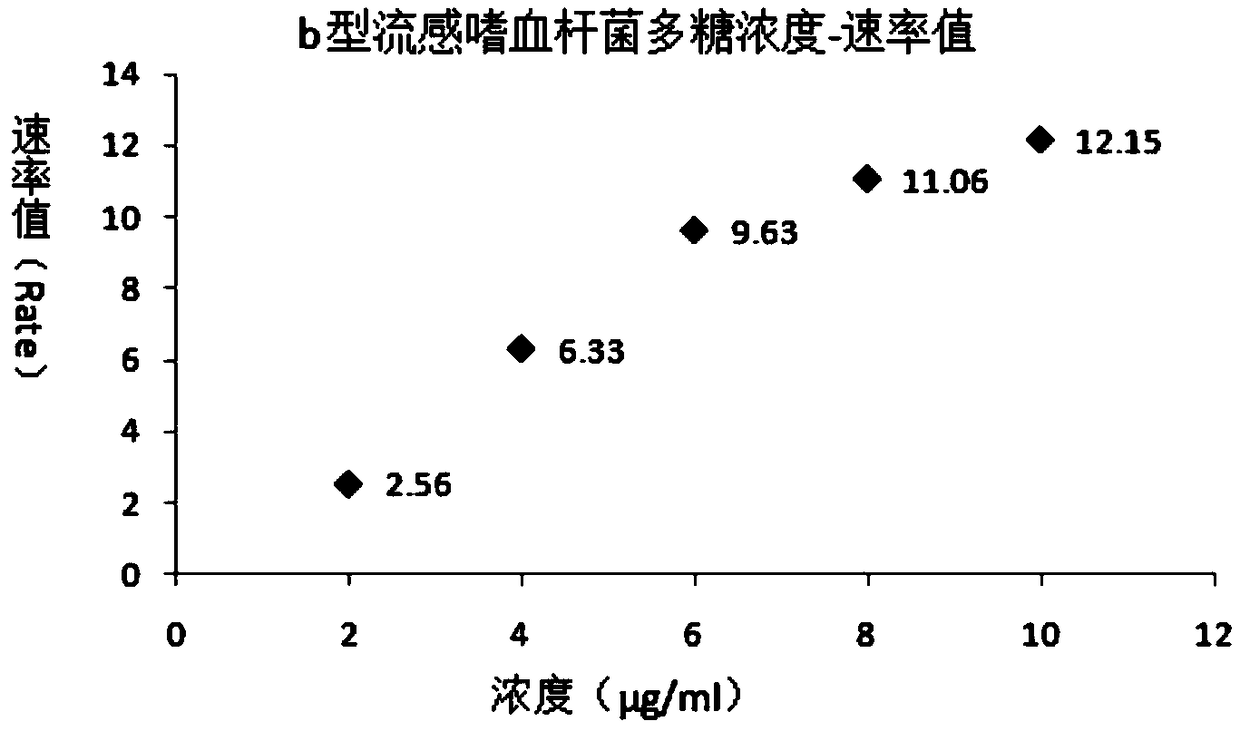
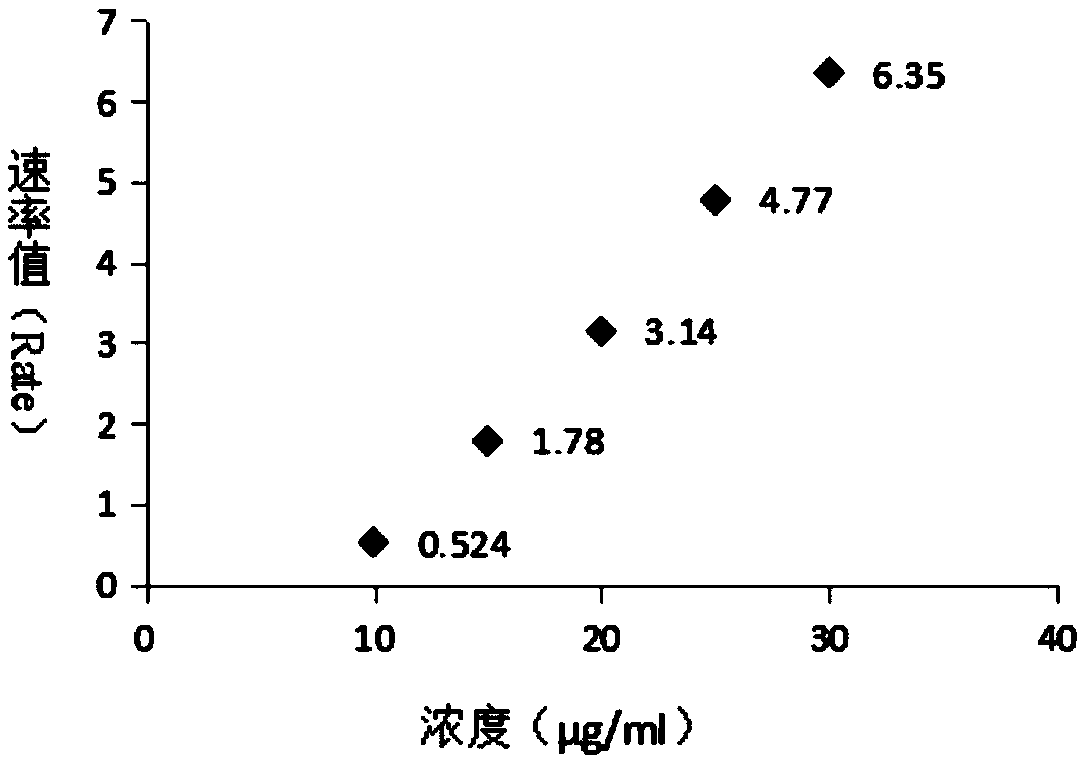
[0105] The present invention provides an immunogenic polysaccharide protein conjugate. The polysaccharide protein conjugate is identified through immunological rate scatter turbidity, and the polysaccharide in the polysaccharide protein conjugate is identified through immunological rate scatter turbidity. The carrier protein in the polyglycoprotein conjugate is identified through immune rate scatter turbidity. The immune rate scatter turbidity includes at least contacting the polyglycoprotein conjugate solution with the corresponding antibody to form an antigen-antibody complex, and then the immune rate scatter ratio Turbidity measures the value of the reaction rate for generating antigen-antibody complexes.
[0106] The further optimized technical scheme of the present invention is that the polysaccharide protein conjugates include pneumococcal capsular polysaccharide protein conjugates, meningococcal capsular polysaccharide protein conjugates, Haemophilus influenzae capsular pol...
Embodiment 2
[0135] The present invention also provides a polysaccharide protein conjugate vaccine. The polysaccharide protein conjugate vaccine is identified through immune rate scatter turbidity, and the polysaccharide in the polysaccharide protein conjugate vaccine is identified through immune rate scatter turbidity. The carrier protein in the polysaccharide protein-conjugated vaccine is identified by immuno-rate scattering turbidity, which includes at least contacting the polysaccharide-protein-conjugated vaccine solution with the corresponding antibody to form an antigen-antibody complex, and then the immuno-rate scattering turbidity Determine the reaction rate value of the antigen-antibody complex.
[0136] The further optimized technical scheme of the present invention is that the polysaccharide protein conjugate vaccine includes pneumococcal capsular polysaccharide protein conjugate vaccine, meningococcal capsular polysaccharide protein conjugate vaccine, Haemophilus influenzae capsula...
Embodiment 3
[0166] Sample source: Meningococcal serogroups A, C, Y, W135 capsular polysaccharides, Haemophilus influenzae serotype b capsular polysaccharides, tetanus toxoid reference materials were purchased from NIBSC (British National Institute for Biological Products), pneumonia Cocci serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F capsular polysaccharide standards were purchased from ATCC, meningococcal serogroups A, C, Y, W135 Antisera were purchased from BD Bio, Haemophilus influenzae serotype b antisera were purchased from BD Bio, pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F antisera were purchased from the Danish National Serum Institute.
[0167] (1) Prepare the following polysaccharide protein conjugate stock solution and polysaccharide protein conjugate vaccine according to the company's another invention patent CN201010129404.2 and Chinese patent CN201610622999.2. Chinese patent CN201310511943.6 published methods: Group A meningococca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com