Modified kgm lecithin-loaded nmn transdermal protosomes, preparations, preparation process and application
A technology of transdermosome and ethosome, which is applied in the field of preparations and biopolymer material modified KGM lecithin loaded with NMN transdermal ethosome, which can solve the problems of short biological half-life, limited potential application, frequent administration, etc. Achieve the effects of high encapsulation rate, stable thermodynamic properties, and fast transdermal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
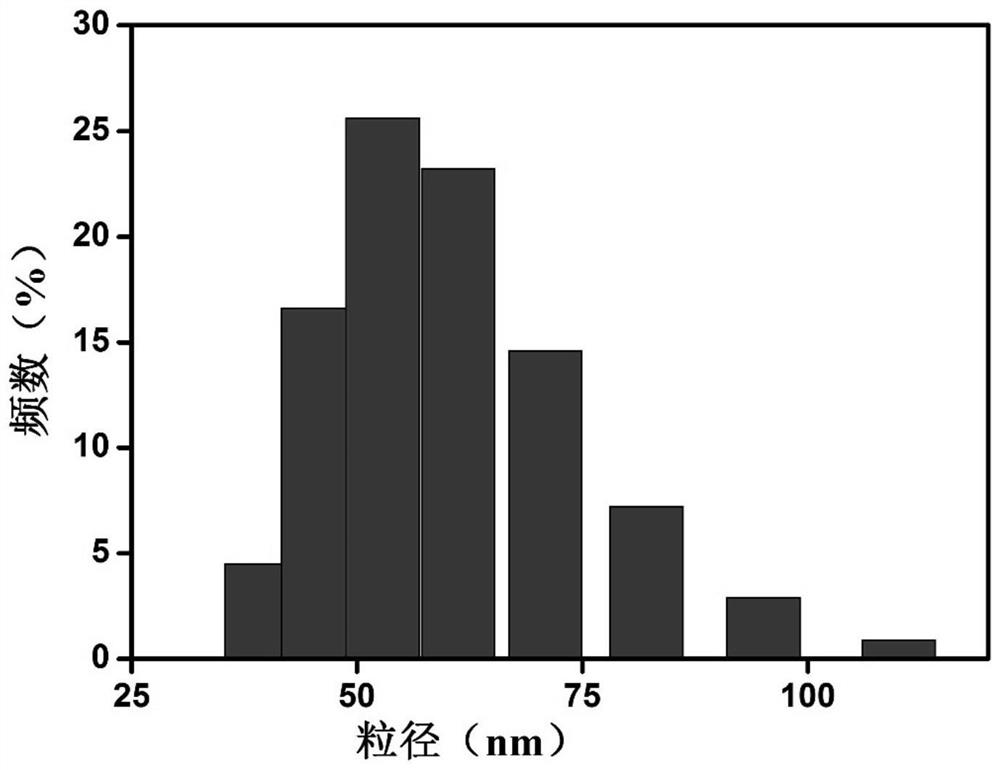
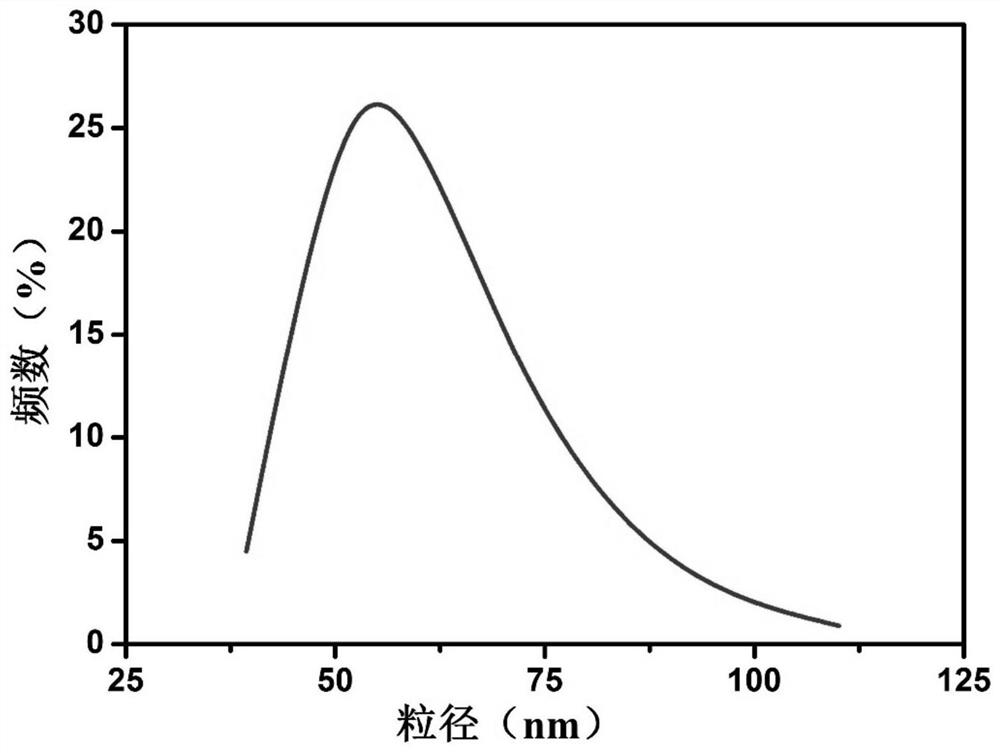
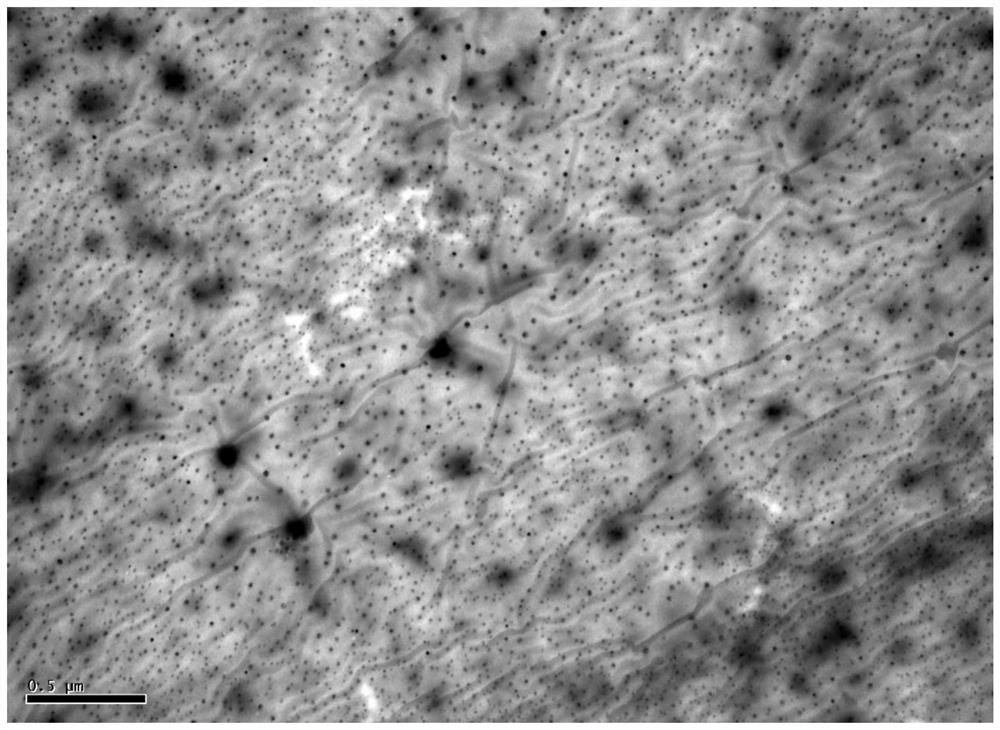
[0043] This embodiment provides a modified KGM lecithin-loaded NMN transdermal ethosome, which consists of the following components in terms of mass percentage: 0.03% of NMN, 0.1% of konjac glucomannan, and 1% of phospholipid , cholesterol 0.05%, stabilizer 0.1%, antioxidant 0.15%, low molecular weight alcohol 5%, and the balance is water. Wherein, the konjac glucomannan (KGM) includes an equal amount of quaternized konjac glucomannan (QKGM) and carboxymethyl konjac glucomannan (CKGM), the phospholipid is soybean lecithin, and the The stabilizer is dicetyl phosphate, the antioxidant is vitamin C, and the low molecular weight alcohol is glycerol.
[0044] The modified KGM lecithin-loaded NMN transdermal protosome gel can be used in medicines for treating Alzheimer's disease, Parkinson's disease and muscular dystrophy, as well as in anti-oxidation and anti-aging preparations.
[0045] This embodiment also provides a modified KGM lecithin-loaded NMN transdermal etosome gel, whic...
Embodiment 2
[0058] This embodiment provides a modified KGM lecithin-loaded NMN transdermal ethosome, which consists of the following components in terms of mass percentage: 1% NMN, 10% konjac glucomannan, 10% phospholipid, Cholesterol 1%, stabilizer 0.5%, antioxidant 1%, low molecular weight alcohol 50%, and the balance is water. Wherein, the konjac glucomannan (KGM) includes quaternized konjac glucomannan (QKGM) and chitosan (CS), the mass ratio of the two is 1:2, and the phospholipid is egg yolk lecithin, Described stabilizer is the mixture of phospholipid phthaloglycerol and phosphoric acid ester, and the mass ratio of the two is 1:1, and described antioxidant is the mixture of vitamin E, di-tert-butyl-p-cresol, and the mass ratio of the two is 1:1: 2. The low molecular weight alcohol is ethanol.
[0059] The modified KGM lecithin-loaded NMN transdermal protosome gel can be used in medicines for treating Alzheimer's disease, Parkinson's disease and muscular dystrophy, as well as in an...
Embodiment 3
[0069] This embodiment provides a modified KGM lecithin-loaded NMN transdermal ethosome, which consists of the following components in terms of mass percentage: NMN 5%, konjac glucomannan 5.5%, phospholipid 6% , cholesterol 0.5%, stabilizer 0.3%, antioxidant 0.45%, low molecular weight alcohol 30%, and the balance is water. Wherein, the konjac glucomannan (KGM) comprises a mixture of oxidized konjac glucomannan (OKGM) and quaternized konjac glucomannan (QKGM), the mass ratio of the two is 1:2, and the phospholipid It is hydrogenated soybean lecithin, the stabilizer is phosphatidyl phthaloserine, the antioxidant is a mixture of malic acid and β-carotene, the mass ratio of the two is 1:1, and the low molecular weight alcohol is n-butanol and A mixture of ethanol, the volume ratio of the two is 1:2.
[0070] The modified KGM lecithin-loaded NMN transdermal protosome gel can be used in medicines for treating Alzheimer's disease, Parkinson's disease and muscular dystrophy, as well...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com