Method for preparing soft chewing dosage form for drug delivery
A technology of soft chewing and medicine, which is applied in the field of medicine, can solve the problems of high equipment performance requirements, preparation technology and technology that cannot be mass-produced, astringent punching, etc., and achieve the effect of getting rid of restrictions and shackles and facilitating large-scale production and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
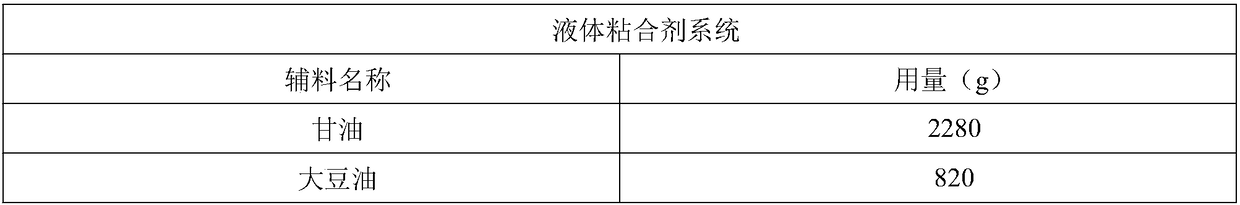
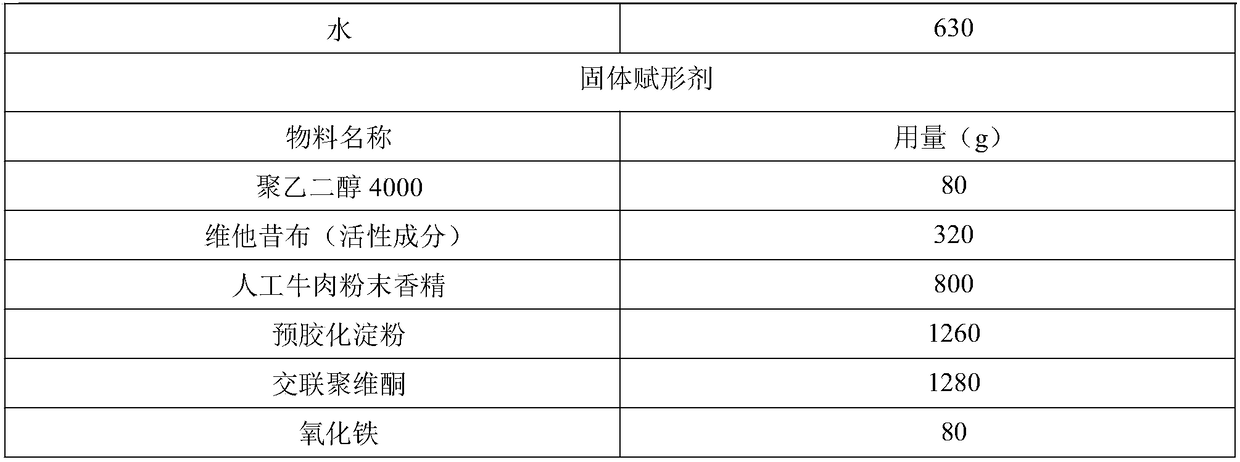
[0030] Example 1: Active Ingredient Containing Vetacoxib - Prescription 1 - Formulation
[0031] The composition of the formula is as follows (4000 batches, specification 80mg):
[0032]
[0033]
[0034] making process:
[0035] Mix the prescribed amount of glycerin, soybean oil and water at room temperature for about 15 minutes to make it uniform; use a wet granulator to mix the prescribed amount of solid excipients at room temperature for about 20 minutes to make it uniform; mix the liquid binder Slowly add to the well-mixed dry auxiliary materials and continue to stir for 10 minutes to obtain wet granules; use a drying oven (non-circulating air, set temperature 25 degrees) to slowly dry the wet granules until the moisture reaches 3-8%, Preferably 4-6%; use nylon sieve or steel sieve to granulate, and the mesh number of the screen is less than 80 mesh (preferably 16-24 mesh); use extruder or molding machine to form, or use tablet press to select appropriate punch Fo...
Embodiment 2
[0046] Example 2: Active ingredient containing benazepril - prescription 2 - formulation
[0047] The composition of the formula is as follows (4000 batches, specification 5mg):
[0048]
[0049]
[0050] Preparation process: with embodiment 1.
[0051] Prescription 2 was carried out palatability study and stability study, the results are as follows:
[0052] (1) Soft Chewing Prescription 2 Dog / Cat Palatability Test
[0053] The dog / cat palatability test is shown in the table below. The results showed that the palatability of formulation 2 in both dogs and cats was very good (greater than 80%).
[0054]
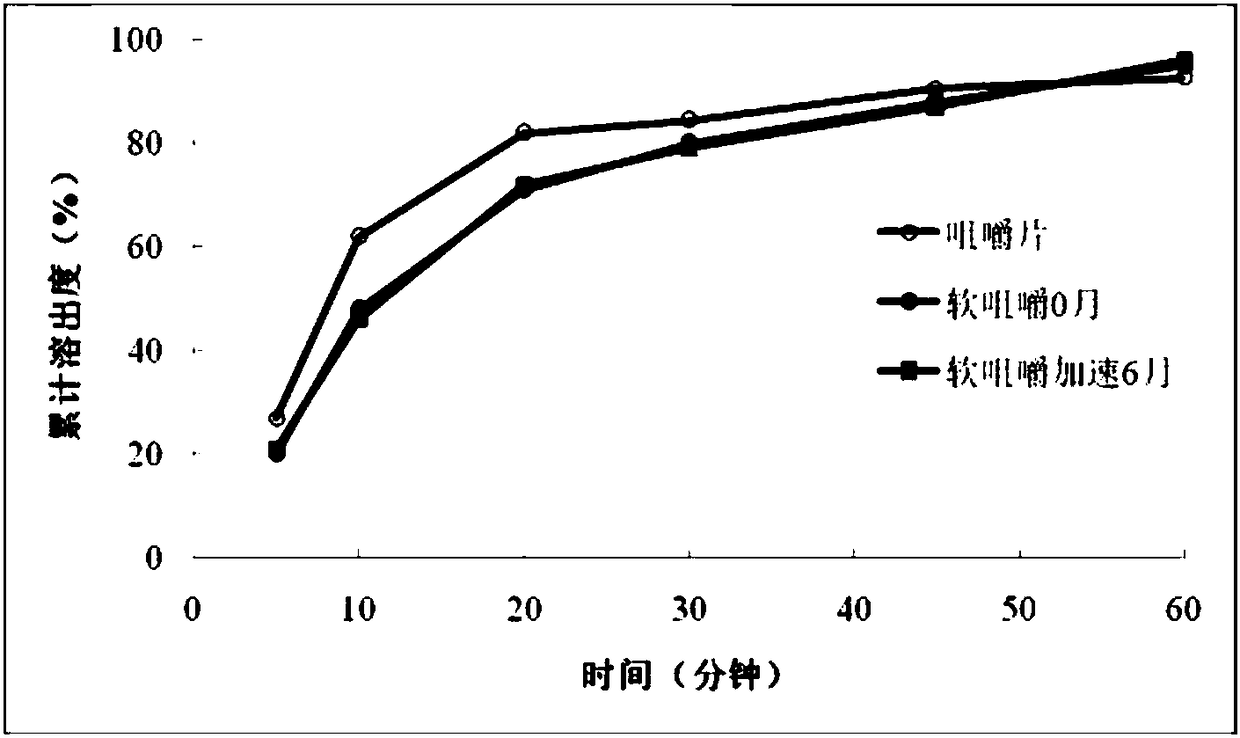
[0055] (2) Stability test of soft chewing prescription 2
[0056] The soft chews of prescription 2 were packaged in bottles and placed under accelerated conditions for 6 months (40°C ± 2°C, RH75% ± 5%) to observe the stability of the soft chews, the results are shown in the table below. The quality of prescription 2 soft chews was stable under accelerated condit...
Embodiment 4
[0058] Example 4: Active ingredients containing anthelmintics or antibiotics - prescriptions 3-8 - preparations
[0059] The formula is composed as follows (4000 batches):
[0060]
[0061]
[0062] Remarks: The active ingredients used in prescriptions 3-5 are compound preparations of praziquantel and milbexime, the active ingredients used in prescriptions 6-8 are preparations of mapoxacin, and the essences used in prescriptions 3-8 are natural chicken flavor, natural Chicken liver flavor, natural bacon flavor, artificial chicken flavor, artificial chicken liver flavor, artificial bacon flavor. Preparation process: with embodiment 1.
[0063] The palatability of prescriptions 3-8 was investigated, and the results are as follows:
[0064] (1) Soft chewing prescription 3-8 dog / cat palatability test
[0065] The dog / cat palatability test is shown in the table below (50 animals). The results showed that the flavors tested had good palatability in both dogs and cats, and ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap