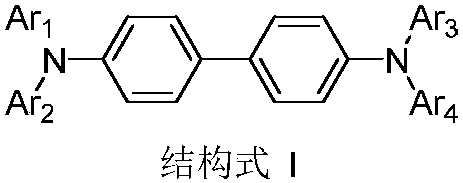
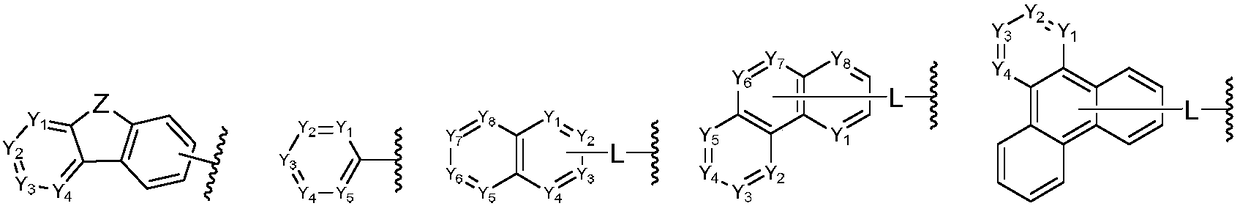
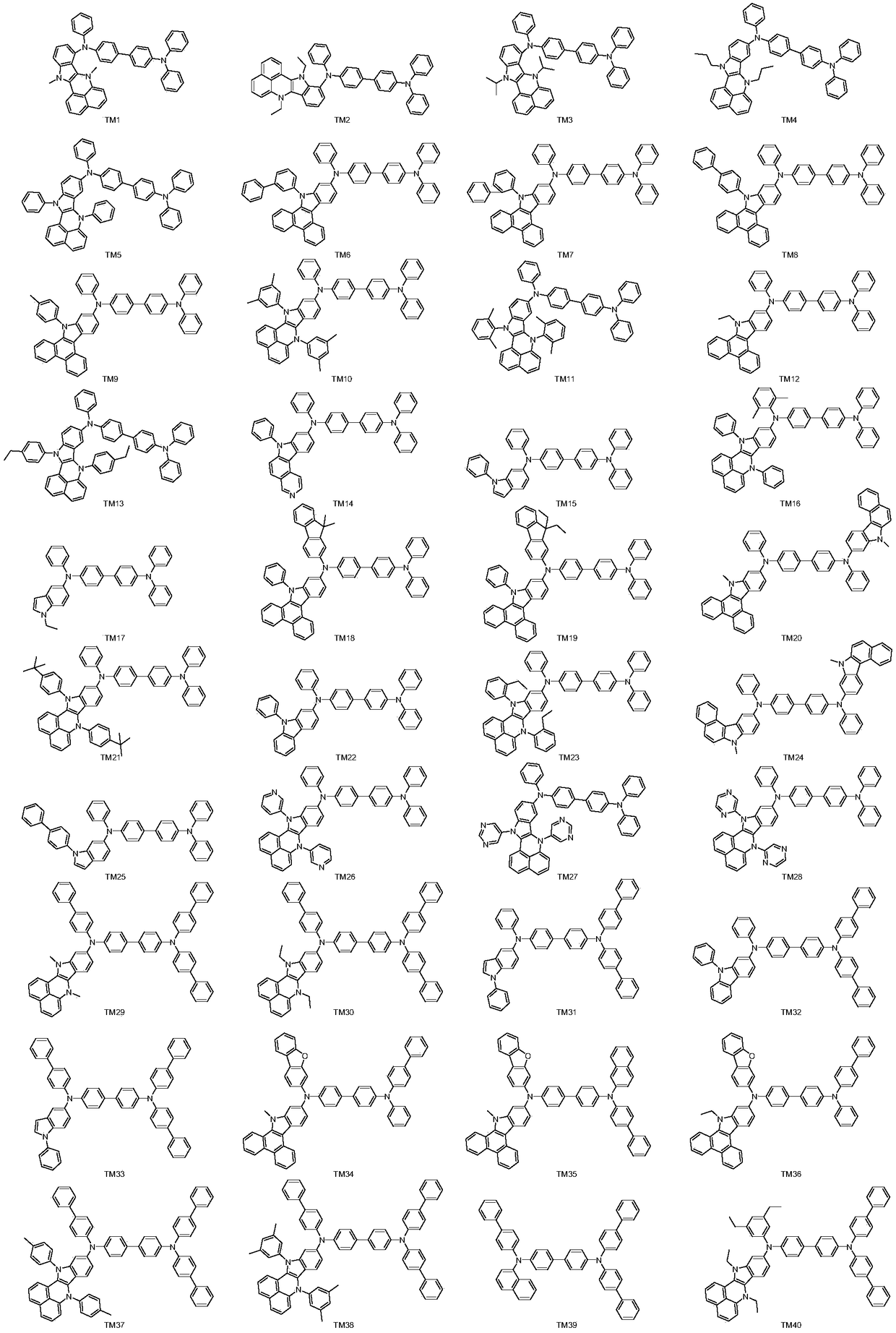
Aromatic amine derivative and organic electroluminescent device thereof
An electroluminescent device, aromatic amine technology, applied in the direction of electro-solid devices, electrical components, organic chemistry, etc., can solve the problem of unable to provide satisfactory light-emitting characteristics, to improve the glass transition temperature and thermal stability, low Effect of driving voltage, large rigid structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: the preparation of compound TM5
[0081] Preparation of Compound A1:
[0082]
[0083] Under argon protection, 5-chloro-1H-indole-2-boronic acid (10.2g, 52.3mmol), iodobenzene (21.3g, 104.7mmol), CuI (5g, 26.2mmol), CuI (5g, 26.2mmol) and Ethylenediamine (1.8ml, 26.2mmol), Cs 2 CO 3 (51.2g, 157.0mmol) and toluene (250ml), the reaction mixture was stirred for one day. The organic phase was extracted with ethyl acetate, the organic phase was concentrated and purified by column chromatography to obtain compound a1 (7.1 g, 50%).
[0084] Under argon protection, 8-bromo-1-naphthylamine (13.3g, 60.0mmol), compound a1 (17.9g, 66mmol), NaOH (7.2g, 180mmol), Pd(PPh 3 ) 4 (3.47g, 3mmol) and THF / H 2 O (80ml / 40ml), the reaction mixture was stirred at 80°C for 12 hours. After the reaction was completed, it was cooled to room temperature, extracted with toluene, and the organic phases were combined. The organic phase was washed with saturated brine, and the org...
Embodiment 2
[0097] Embodiment 2: the preparation of compound TM13
[0098] The iodobenzene in Example 1 was replaced by equimolar 1-ethyl-4-iodobenzene, and the other steps were the same as in Example 1 to obtain compound TM13 (6.6 g, 75%). Mass spectrum m / z: theoretical value: 875.13; found value: 877.31. Theoretical element content (%)C 64 h 50 N 4 :, 87.84; H, 5.76; N, 6.40; Measured element content (%): , 87.81; H, 5.83; N, 6.36. The above results confirmed that the obtained product was the target product.
[0099]
Embodiment 3
[0100] Embodiment 3: the preparation of compound TM61
[0101] The diphenylamine in Example 1 was replaced by equimolar N-phenyl-4-benzidine, and other steps were the same as in Example 1 to obtain compound TM61 (6.4 g, 72%). Mass spectrum m / z: theoretical value: 895.12; found value: 896.73. Theoretical element content (%)C 66 h 46 N 4 : C, 88.56; H, 5.18; N, 6.26; Measured element content (%): C, 88.54; H, 5.23; N, 6.23. The above results confirmed that the obtained product was the target product.
[0102]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com