Paeoniflorin composition for treating gastrointestinal dysfunction or irritable bowel syndrome and application thereof
A technology for irritable bowel syndrome and gastrointestinal dysfunction, which is applied in the field of natural pharmaceutical compositions to achieve the effects of clear chemical composition, reduced clinical dosage, and improved preparation level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0075] Effect of Composition on Visceral Hypersensitivity in Irritable Bowel Syndrome Model Rats
[0076] 1. Experimental animals and medicines are provided through legal channels. Pinaverium Bromide Tablets 50mg / tablet (Abbott Products SAS) for experiments; paeoniflorin (98%), glycyrrhizic acid (95%), naringin (98%), hesperidin (98%), isoliquiritin for experiments (98%), Liquiritin (98%) and Neohesperidin (98%) were provided by Nantong Feiyu Biotechnology Company. The relevant extracts used in the experiment were prepared by ourselves, paeoniflorin extract (content greater than or equal to 20%), glycyrrhizic acid (content greater than or equal to 20%), naringin (content greater than or equal to 20%), hesperidin (content greater than or equal to 20%), neohesperidin extract (content greater than or equal to 20%), liquiritin (content greater than or equal to 10%), isoliquiritin (content greater than or equal to 5%). The decoction pieces of traditional Chinese medicine used in ...
experiment example 2
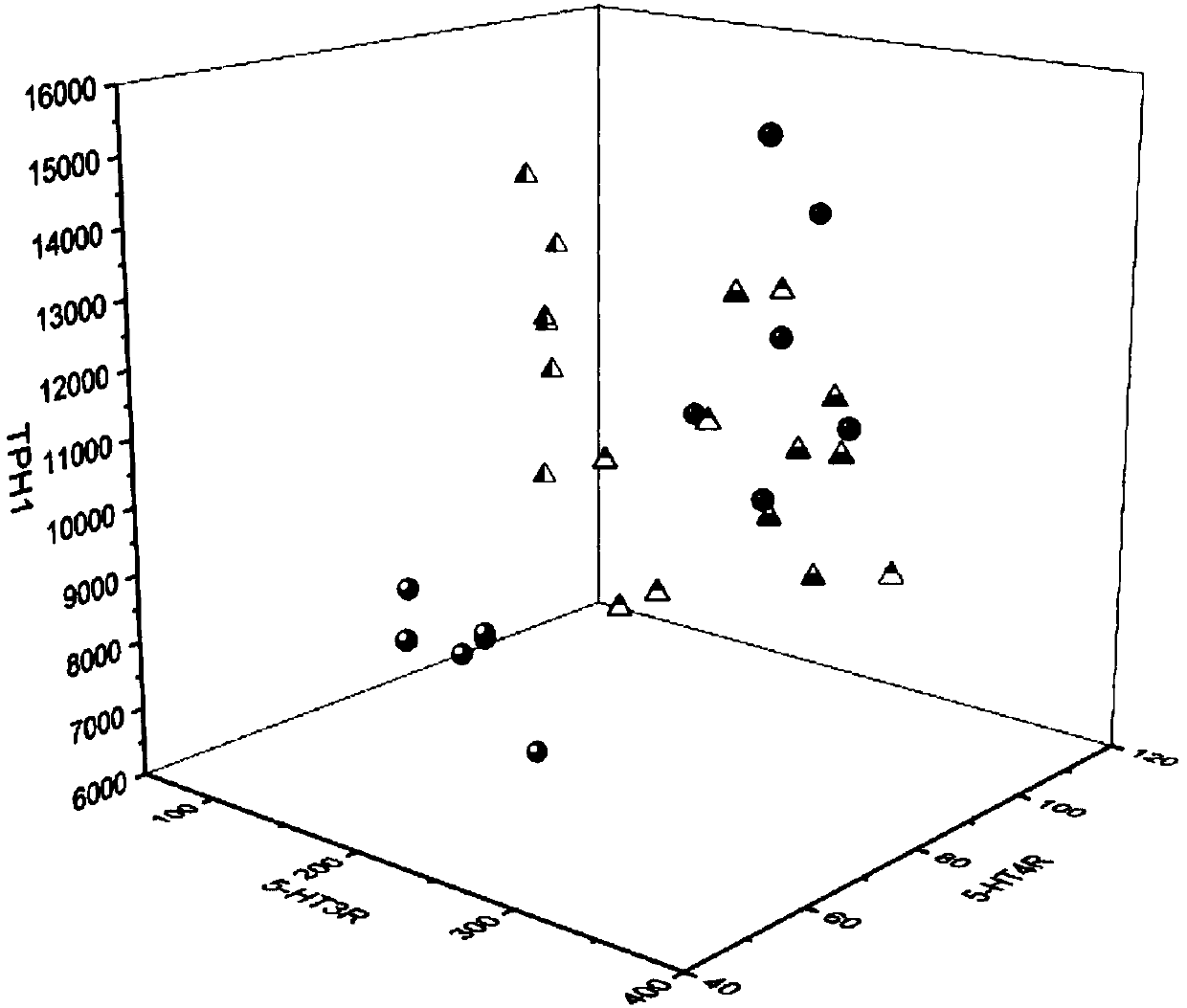
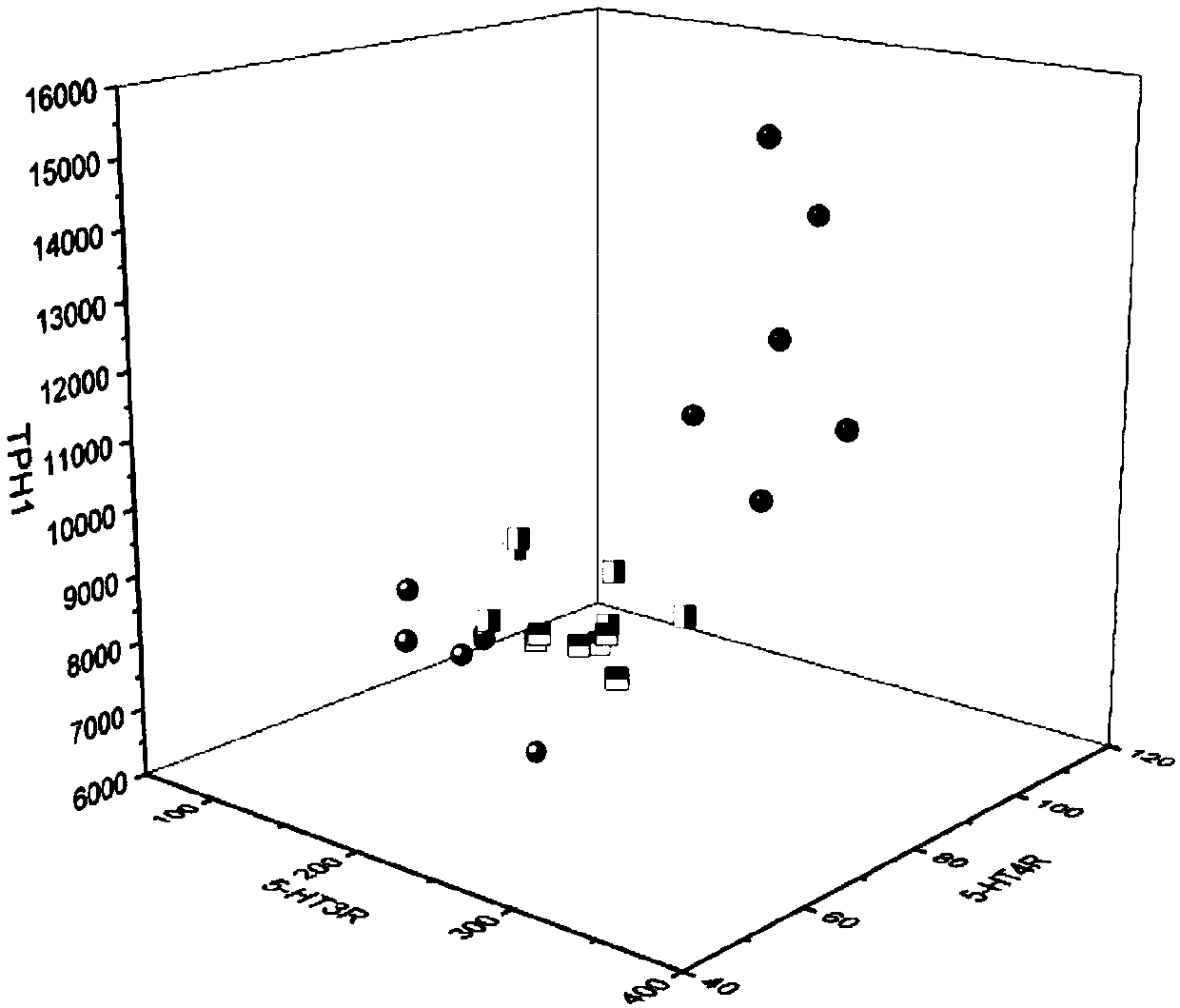
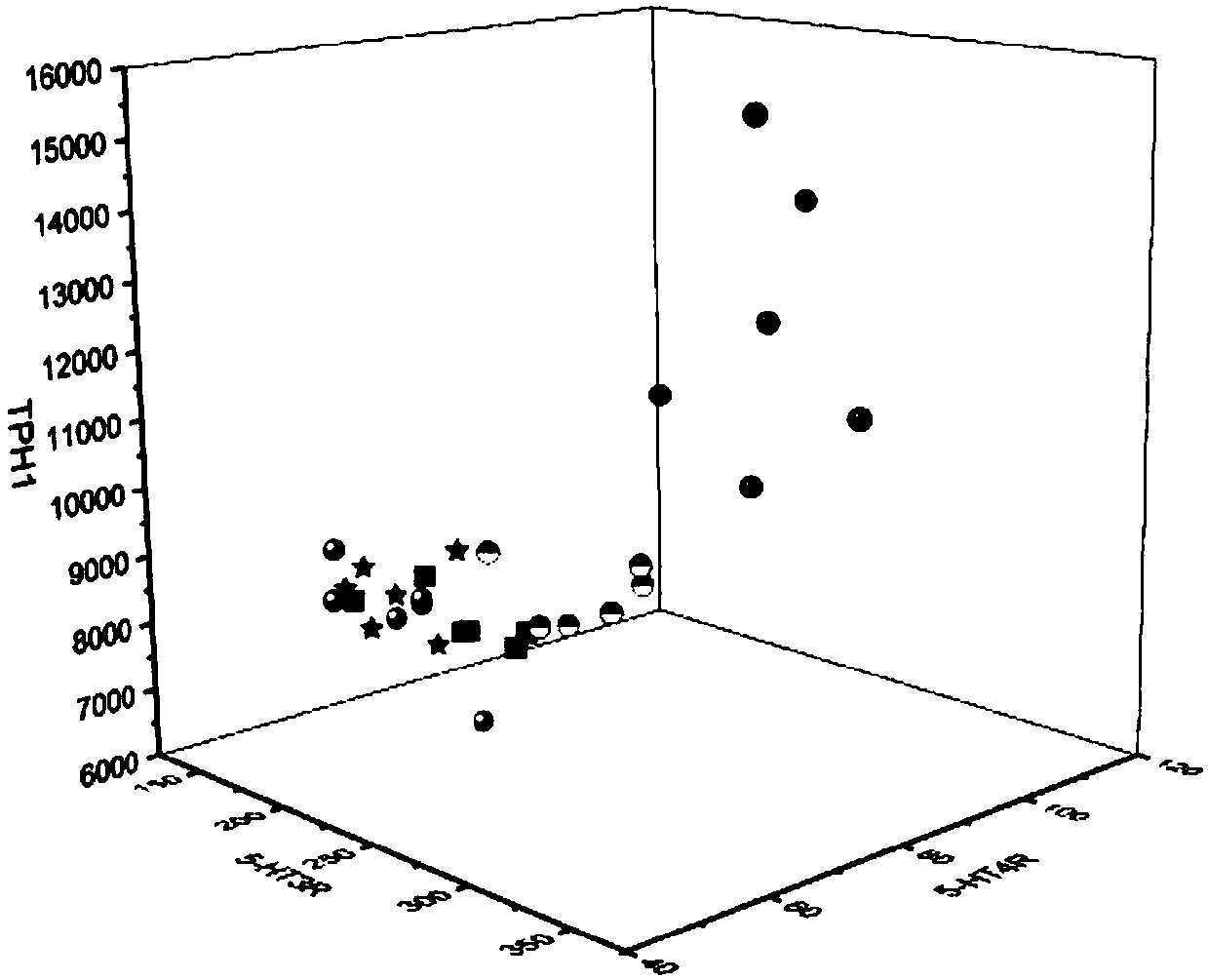
[0127] Effects of the composition on 5-HT signaling pathway and TRPV1 in visceral hypersensitive rats.
[0128] 1. Experimental animals, medicines, modeling, and test product preparation: the same as in Experimental Example 1.
[0129] 2. Experimental method
[0130] Rat colon tissue sample collection: 2 hours after the last gavage, the rats were executed by decapitation, and 3 segments of the colon 3 cm above the anus were taken, each segment 1-2 cm, washed with normal saline and PBS buffer solution (pH 7.4) respectively , blotted dry with filter paper, accurately weighed, quickly frozen with liquid nitrogen, and stored at -80°C for later use.
[0131]5-HT, 5-HT3R, 5-HT4R, TPH1, and SERT protein expression levels in rat colon were measured by enzyme-linked immunosorbent assay (ELISA). The rat colon tissue pieces were washed with PBS buffer, and the tissue was homogenized by adding appropriate saline. slurry, and centrifuged to obtain the supernatant. According to the instr...
experiment example 3
[0155] The adjustment of experimental example 3 to the gastrointestinal dysfunction rat caused by reserpine
[0156] 1. Animals and reagents are the same as in Experimental Example 1.
[0157] 2. Animal grouping and dosage: (1) Normal group was injected intraperitoneally with equal volume of normal saline, and at the same time fed with equal volume of distilled water; (2) Model group: intraperitoneally injected with reserpine 0.5 mg / kg, and simultaneously fed with equal volume of distilled water (3) Positive group: intraperitoneal injection of reserpine 0.5mg / kg, and at the same time, 50 mg / kg of Suliqenone was administered; (4) sample group: intraperitoneal injection of reserpine 0.5mg / kg, and simultaneously, the test samples were administered (with experimental example one). Each group was administered continuously for two weeks. The feces and signs of the rats were observed at any time, and the body weight and food intake of the rats were weighed every day. The following...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com