Method for catalyzing oxicracking of aryl ethers of lignin model
A technology of oxidative cracking and aryl ether, which is applied in the direction of chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as poor selectivity of oxidative bond breaking, complicated preparation of oxidants, and less oxidative cracking reactions. Achieve the effects of less catalyst consumption, good selectivity for oxidative bond breaking, and highly selective oxidative cracking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
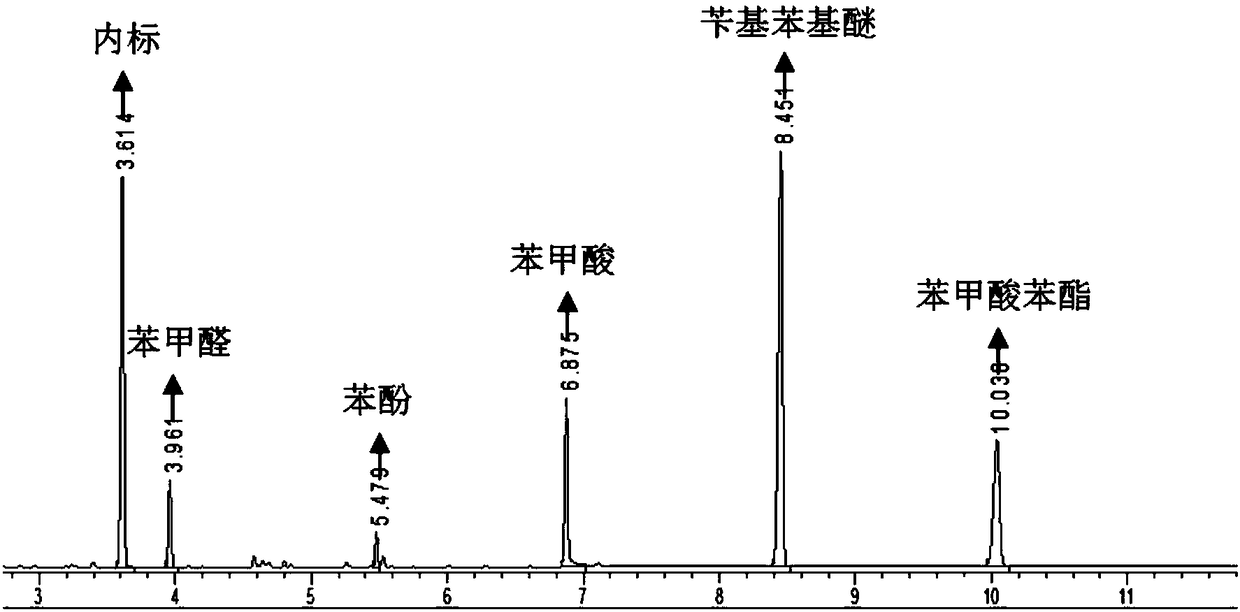
[0013] Add 2.5mmol of benzyl phenyl ether, 0.125mmol of vanadyl sulfate, 0.125mmol of copper nitrate, and 2mL of ethylene glycol dimethyl ether into a 30mL reaction kettle, fill it with oxygen to 1.0MPa, and raise the temperature to 100°C under constant stirring. And kept for 10h, cooled to room temperature. Use the internal standard method to analyze by gas chromatography, and calculate the conversion rate of benzyl phenyl ether and the selectivity of the oxidative bond breaking product according to the following formula.
[0014] Conversion rate [mol%]=(A 0 -A) / A 0 ×100%
[0015] Selectivity [mol%] = B i / (A 0 -A)×100%
[0016] In the formula, A 0 Be the amount of substance [mol] that adds benzyl phenyl ether before the reaction, A is the amount of substance [mol] of benzyl phenyl ether after the reaction, B i It is the amount [mol] of substances that generate benzaldehyde, phenol, benzoic acid, and phenyl benzoate after the reaction. Bond breaking selectivity [mol%]...
Embodiment 2
[0019] Add 2.5mmol of benzyl phenyl ether, 0.075mmol of vanadyl sulfate, 0.075mmol of zinc nitrate, and 2mL of ethylene glycol dimethyl ether into a 30mL reaction kettle, fill it with oxygen to 1.0MPa, and raise the temperature to 120°C under constant stirring. And kept for 10h, cooled to room temperature. Carry out product analysis by embodiment 1 method;
[0020] The conversion rate of benzyl phenyl ether was calculated to be 82%, and the bond breaking selectivity was 80%.
Embodiment 3
[0022] Add 2.5mmol of phenylphenetole, 0.125mmol of vanadyl acetylacetonate, 0.125mmol of copper nitrate, and 2mL of acetic acid into a 30mL reaction kettle, fill it with oxygen to 1.5MPa, raise the temperature to 100°C under constant stirring, and keep it for 6h, then cool to room temperature. Carry out product analysis by embodiment 1 method;
[0023] The conversion rate of phenylphenetole was calculated to be 68%, and the bond breaking selectivity was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com