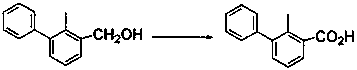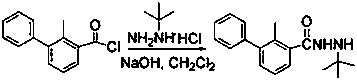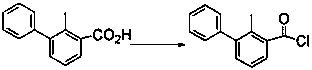N-(2-methyl-3-phenylbenzoyl)-N'-tert-butylhydrazine and synthesis method thereof
A technology of phenylbenzoic acid and methyl, applied in the field of N-(2-methyl-3-phenylbenzoyl)-N'-tert-butylhydrazine and its synthesis, can solve the problem of short residual effect time and toxicity. Low water solubility, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Preparation of compound 2 (2-methyl-3-phenylbenzoic acid)
[0018] 40 g CrO 3 Pour into a volumetric flask containing 120 mL of water, and add 34.5 mL of concentrated H 2 SO 4 , shake well, and dilute with water to 150 mL to obtain Jones reagent; add 30 g of 2-methyl-3-phenylbenzyl alcohol, 32 mL of water, and 200 mL of acetone to a three-neck flask, place in an ice-water bath, and add 90 mL of Jones reagent dropwise. Reagents, control the reaction temperature at 25°C, react for 6 h, track the reaction with thin-layer chromatography, dichloromethane as the developer, wait for the disappearance of the substrate point and the aldehyde point, stop the reaction, and the reaction takes 6 h. Acetone was removed under reduced pressure, a large amount of precipitate was produced, suction filtered, the filter cake was washed with water until white, and dried to obtain 30 g of crude product with a yield of 99%. The crude product was dissolved in ethanol (the amount of...
Embodiment 2
[0019] Example 2 Synthesis of compound 3 (2-methyl-3-phenylbenzoyl chloride)
[0020] Add 5 g of compound 2 into a three-necked flask, add 1-2 drops of DMF, then add 10 mL of toluene, install a tail gas absorption device, raise the temperature to 80 °C, and then add 10 mL of SOCl 2 , after the dropwise addition, continue the constant temperature reaction for 1 h, cool to room temperature, and then remove excess SOCl 2 , the product compound 3 was obtained, which was directly used in the next step of synthesis without purification.
Embodiment 3
[0021] Example 3 Synthesis of Compound 4 (N-tert-butyl-N'-(2-methyl-3-phenylbenzoyl)hydrazine)
[0022] Weigh 3.9g of tert-butylhydrazine hydrochloride and 40 mL of dichloromethane into a four-neck flask, and under nitrogen protection, add 100 mL of 0.3 M sodium hydroxide aqueous solution dropwise at 0 °C. After the dropwise addition, simultaneously Add compound 3 and 100 mL of 0.3 M sodium hydroxide aqueous solution dropwise, keep the addition of the two synchronously, control the temperature of the whole reaction at 4-5 °C, follow the reaction by thin-layer chromatography, developer: dichloromethane: ethyl acetate ( 2:1), after 1.5 h, the temperature was raised to 30 °C to dissolve all the solids in the reaction system, and then the layers were separated, and the oil layer was separated. , suction filtration, and drying to obtain 5.5 g of the product, melting range: 186-189°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com