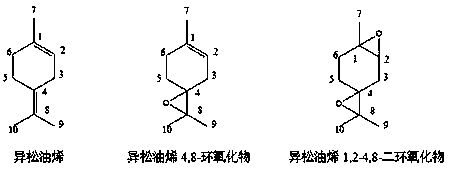
Synthetic method of terpinolene 4,8-epoxide
A technology of terpinene and epoxide, which is applied in the field of synthesis of terpinene 4,8-epoxide, can solve the problems of waste water pollution oxidation selectivity, harsh reaction conditions, difficult catalyst recovery and the like, and achieves oxidation Moderate performance, simple handling, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
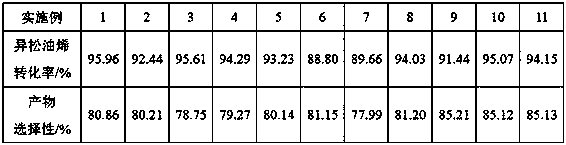
[0027] Mix 10 g of terpinolene raw material (mass fraction 84%), 3 g of 2,2,2-trifluoroacetophenone, 5 g of acetonitrile and 6 g of toluene to obtain a mixture solution, which is transferred to a batch reactor 10g of 50% hydrogen peroxide solution was slowly added dropwise to the mixture solution at 30°C, and the dropping time was 2h. After the dropwise addition, continue to stir and react for 9 hours, then let it stand, collect the organic phase and the water phase after the mixture is separated, add 9 g of sodium sulfite solution (mass fraction 15%) to the organic phase, stir for 30 minutes, and remove the remaining hydrogen peroxide. The remaining organic phase was sampled for chromatographic analysis.
Embodiment 2
[0029] Mix 100 g of terpinolene raw material (mass fraction 84%), 25 g of 2,2,2-trifluoroacetophenone, 50 g of acetonitrile and 50 g of toluene to obtain a mixture solution, which is transferred to a batch reactor 180g of 30% hydrogen peroxide solution was slowly added dropwise to the mixture solution at 35°C for 2 hours. After the dropwise addition, continue to stir and react for 9 hours, then stand still, collect the organic phase and the water phase after the mixture is separated, add 92.4g of sodium sulfite solution (mass fraction 15%) to the organic phase, stir for 30 minutes, and remove the remaining hydrogen peroxide , and the remaining organic phase was sampled for chromatographic analysis.
Embodiment 3
[0031] Hexafluoroacetone was used to replace the 2,2,2-trifluoroacetophenone used in Example 1, and the addition amount was 2.9 g, and the rest of the steps were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com