Application of quercetin in preparing product for treating cholestasis
A quercetin and product technology, applied in the field of medicine, can solve the problems such as the use of quercetin has not been reported yet, and achieve the effects of treating cholestasis and promoting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The therapeutic effect of embodiment 1 quercetin on estrogen-induced rat cholestasis
[0066] The rat model of chronic endogenous cholestasis was established by subcutaneously injecting ethinyl estradiol propylene glycol solution (EE, 2 mg / kg / d) into Wistar male rats continuously for 14 days. Simultaneously with modeling, normal saline, quercetin (55.62mg / kg / d), and positive drug UDCA (60mg / kg / d) were given by intragastric administration for 14 days.
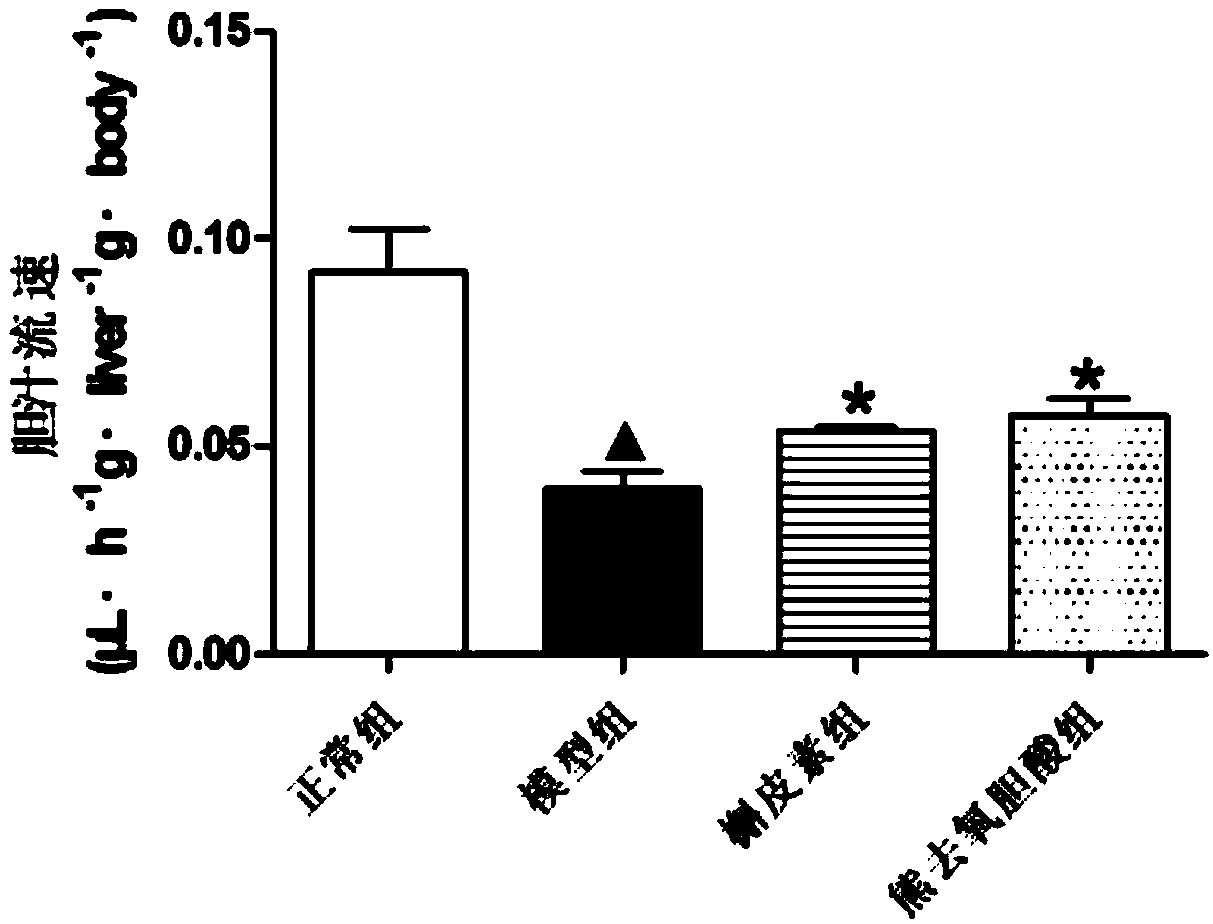
[0067] After 14 days, the rats were anesthetized and fixed with ether, and after bile duct intubation, the bile was collected with a PE tube for 1 hour, and the flow rate of the bile was measured. The test results are as follows: figure 1 shown.
[0068] Bile flow rate mainly reflects whether the uptake and efflux of bile in the hepatobiliary system is normal. After subcutaneous injection of EE in rats for 14 days, the bile flow rate of the rats in the model group was significantly lower than that of the control group (...
Embodiment 2
[0069] Example 2 Effect of Quercetin on Serum Biochemical Indexes in Cholestatic Rats
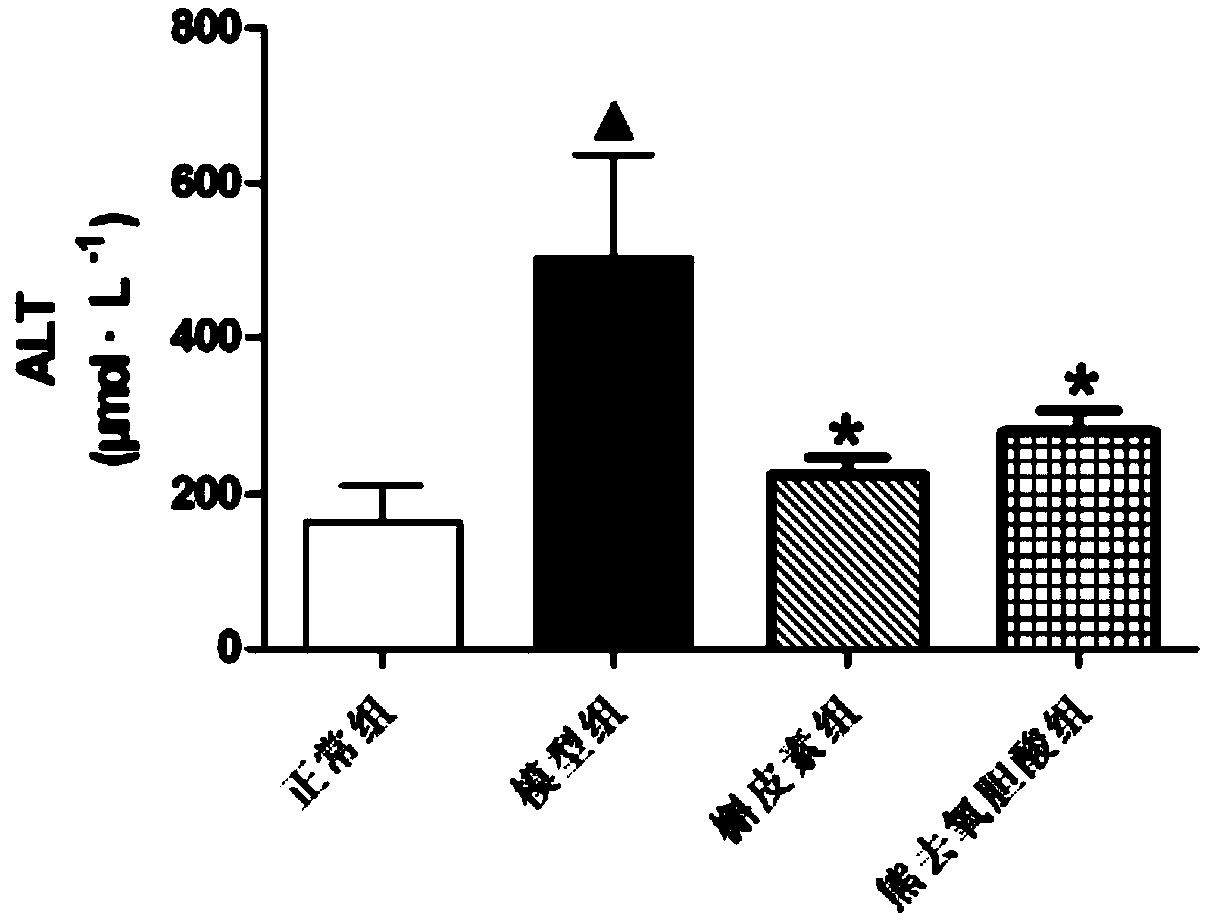
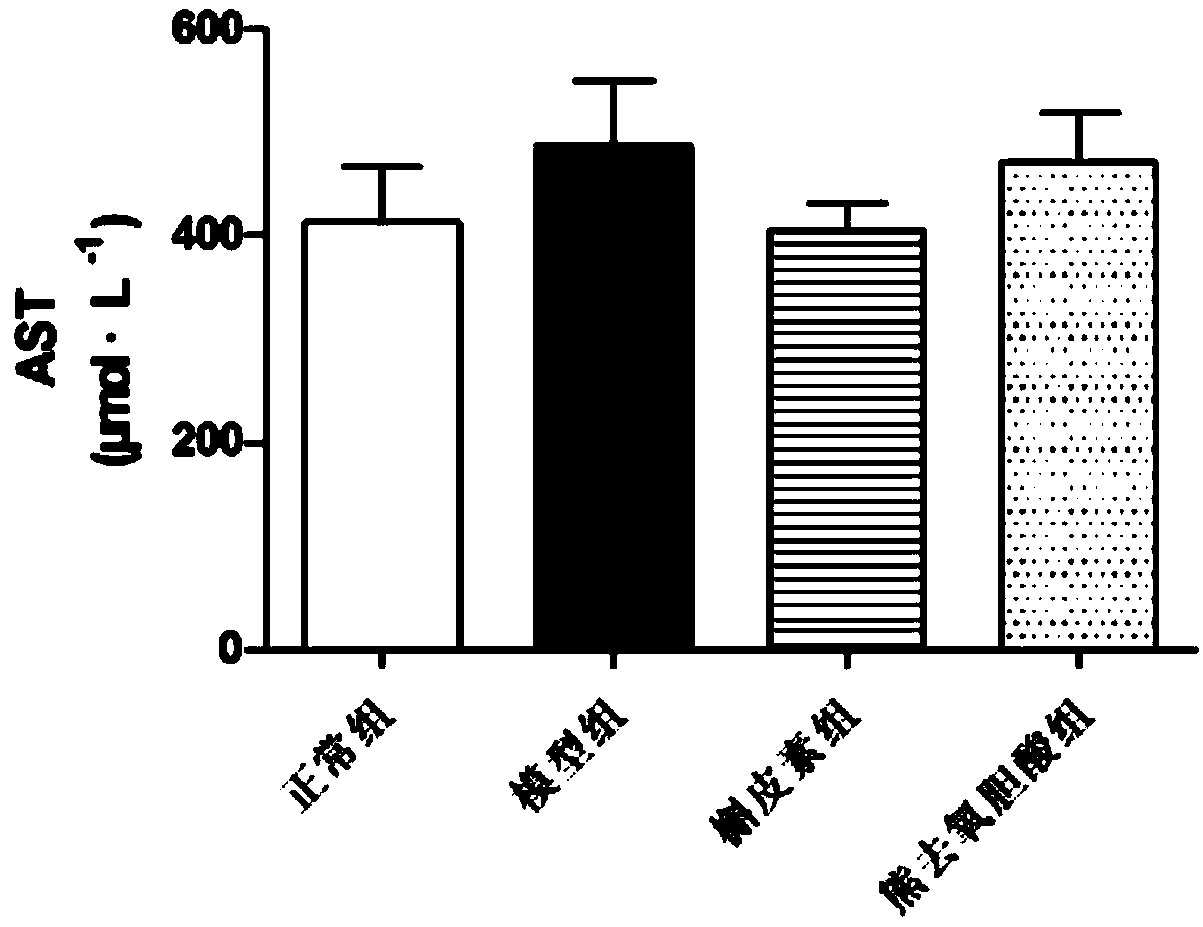
[0070] After the rats were anesthetized with ether, blood was taken from the abdominal aorta, and placed in a test tube rinsed with a coagulant. After centrifugation, the upper serum was collected, and the collected serum samples were put into a biochemical analyzer for detection. Measure the contents of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TBIL) and total bile acid (TBA) in rat serum respectively, and its test results are as follows: Figure 2-6 shown.
[0071] In this experiment, 14 days after rats were subcutaneously injected with EE, TBA and TBIL in the model group were significantly higher than those in the control group (P<0.05), indicating that the rat cholestasis model was successfully established.
[0072] Compared with the model group, the TBA content of each administration group decreased significantly (P0...
Embodiment 3
[0075] Example 3 Effect of quercetin on liver tissue structure in cholestasis rats
[0076] After blood collection from the abdominal aorta of rats, some liver tissues were soaked in 10% formaldehyde solution and fixed for 24 hours. The samples were dehydrated and embedded in paraffin. After sectioning, they were stained with HE and sealed with gum to make pathological slices of rat liver tissue. , placed the prepared pathological sections under an optical microscope to observe the changes in the rat liver tissue structure, the test results are as follows Figure 7-10 shown.
[0077] In this experiment, the liver tissue structure and cell morphology in the control group were normal. After 14 days of subcutaneous injection of EE, compared with the control group, capillary bile duct hyperplasia, inflammatory cell infiltration, venous dilation and congestion, and local liver cell necrosis appeared in the liver tissue of the model group, indicating that the rat cholestasis model ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com