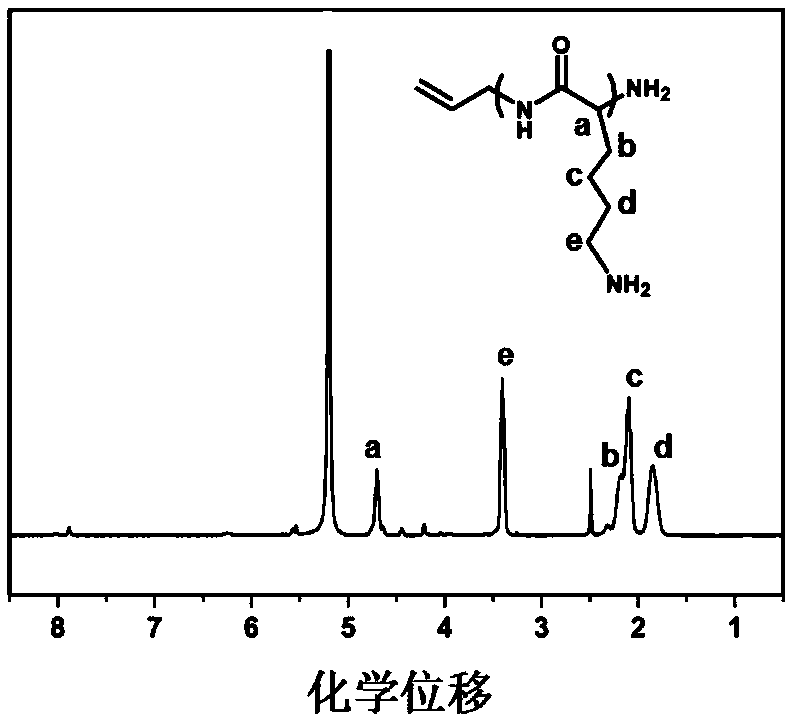
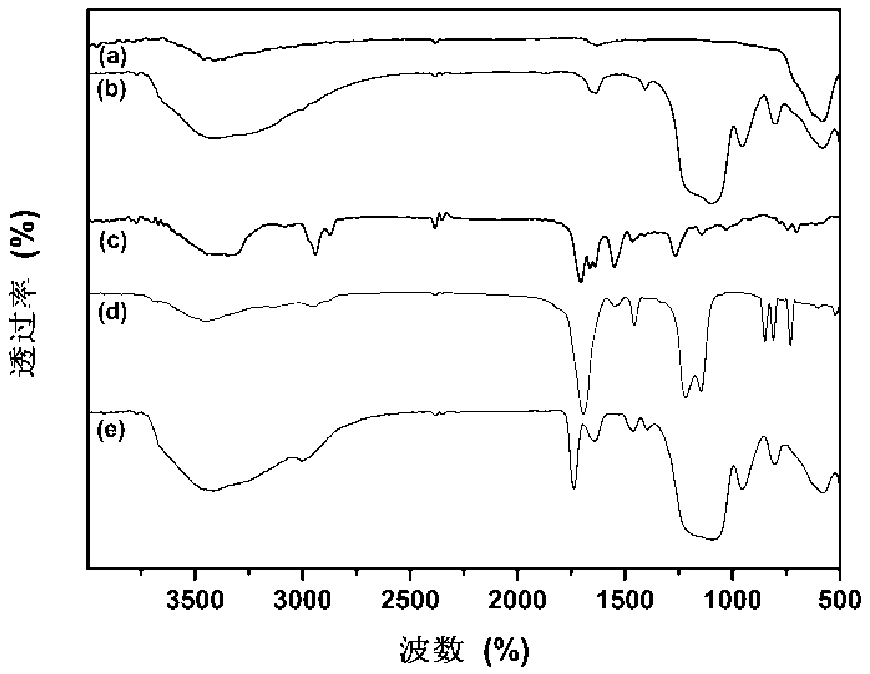
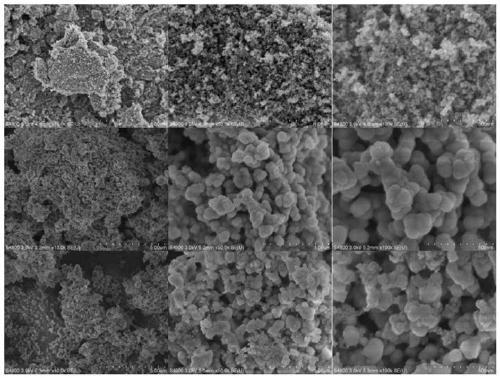
Preparation method of cationic polyamino acid magnetic adsorbent
A magnetic adsorbent, polyamino acid technology, applied in chemical instruments and methods, adsorption water/sewage treatment, alkali metal oxides/hydroxides, etc., to achieve good structural stability and improve adsorption efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] FeCl 3 ·6H 2 O 2.0 g and FeCl 2 4H 2 Add 0.8 g of O into 40 mL of deionized water, and stir at a speed of 700 r / min to raise the temperature to 80°C under nitrogen gas. After stirring for another 30 minutes, add ammonia water to adjust the pH of the solution to 9, raise the temperature to 90°C, and continue the reaction for 120 minutes. After the reaction, the product was cooled to room temperature, centrifuged, and the upper liquid was removed, washed with 100mL ethanol and deionized water, and repeated 5 times; the obtained product was vacuum-dried at 50°C for 3h to obtain Fe 3 o 4 Magnetic nanoparticles, finally ground for use.
[0038] Fe 3 o 4Put 0.40g of magnetic nanoparticles into a mixed solvent of 40mL deionized water and 5mL ethanol, ultrasonically disperse for 10min, add 2mL ammonia water and 1.3mL orthosilicate ethyl ester, stir and react at room temperature at a speed of 600r / min for 3.5h, and react After centrifugation, remove the upper liquid, wash...
Embodiment 2
[0042] FeCl 3 ·6H 2 O 2.2g and FeCl 2 4H 2 O 0.9 g was added to 50 mL of deionized water, under nitrogen, stirred at a speed of 700 r / min and heated to 80 ° C, after stirring for 30 min, ammonia water was added to adjust the pH of the solution to 9, the temperature was raised to 90 ° C, and the reaction was continued for 120 min. After the reaction, the product was cooled to room temperature, centrifuged, and the upper liquid was removed, washed with 100mL ethanol and deionized water, and repeated 5 times; the obtained product was vacuum-dried at 50°C for 3h to obtain Fe 3 o 4 Magnetic nanoparticles, finally ground for use.
[0043] Fe 3 o 4 Put 0.40g of magnetic nanoparticles into a mixed solvent of 45mL of deionized water and 6mL of ethanol, ultrasonically disperse for 10min, add 2mL of ammonia water and 1.4mL of ethyl silicate, stir and react at room temperature at a speed of 600r / min for 3.5h, and react After centrifugation, remove the upper liquid, wash 5 times wit...
Embodiment 3
[0047] FeCl 3 ·6H 2 O 2.4g and FeCl 2 4H 2 O 1.0g was added to 60mL deionized water, under nitrogen, stirred at a speed of 700r / min and heated to 80°C, stirred for another 30min, added ammonia water to adjust the pH of the solution to 9, heated to 90°C, and continued to react for 120min. After the reaction, the product was cooled to room temperature, centrifuged, and the upper liquid was removed, washed with 100mL ethanol and deionized water, and repeated 5 times; the obtained product was vacuum-dried at 50°C for 3h to obtain Fe 3 o 4 Magnetic nanoparticles, finally ground for use.
[0048] Fe 3 o 4 Put 0.40g of magnetic nanoparticles into a mixed solvent of 50mL deionized water and 7mL ethanol, ultrasonically disperse for 10min, add 2mL ammonia water and 1.5mL orthosilicate ethyl ester, stir and react at room temperature at a speed of 600r / min for 3.5h, and react After centrifugation, remove the upper liquid, wash 5 times with 100mL deionized water, and vacuum-dry the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com