Composite photocatalyst, preparation method of composite photocatalyst, and photocatalytic hydrogen production method
A technology of composite light and catalyst, applied in the field of photocatalysis, can solve the problems of low solar energy utilization rate, low quantum efficiency, and limited application, and achieve good visible light photocatalytic activity, improve quantum efficiency, and easy-to-source effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0042] Embodiment 2 of the present invention is a method for preparing a composite photocatalyst, including the following steps:
[0043] Step A1, disperse barium niobate nanosheets in the second ultrapure water, and add urea to obtain solution D;
[0044] Step A2, heating the solution D and fully stirring until a dry product is formed;
[0045] Step A3, calcining the dried product, and separating a pale yellow precipitate after cooling;
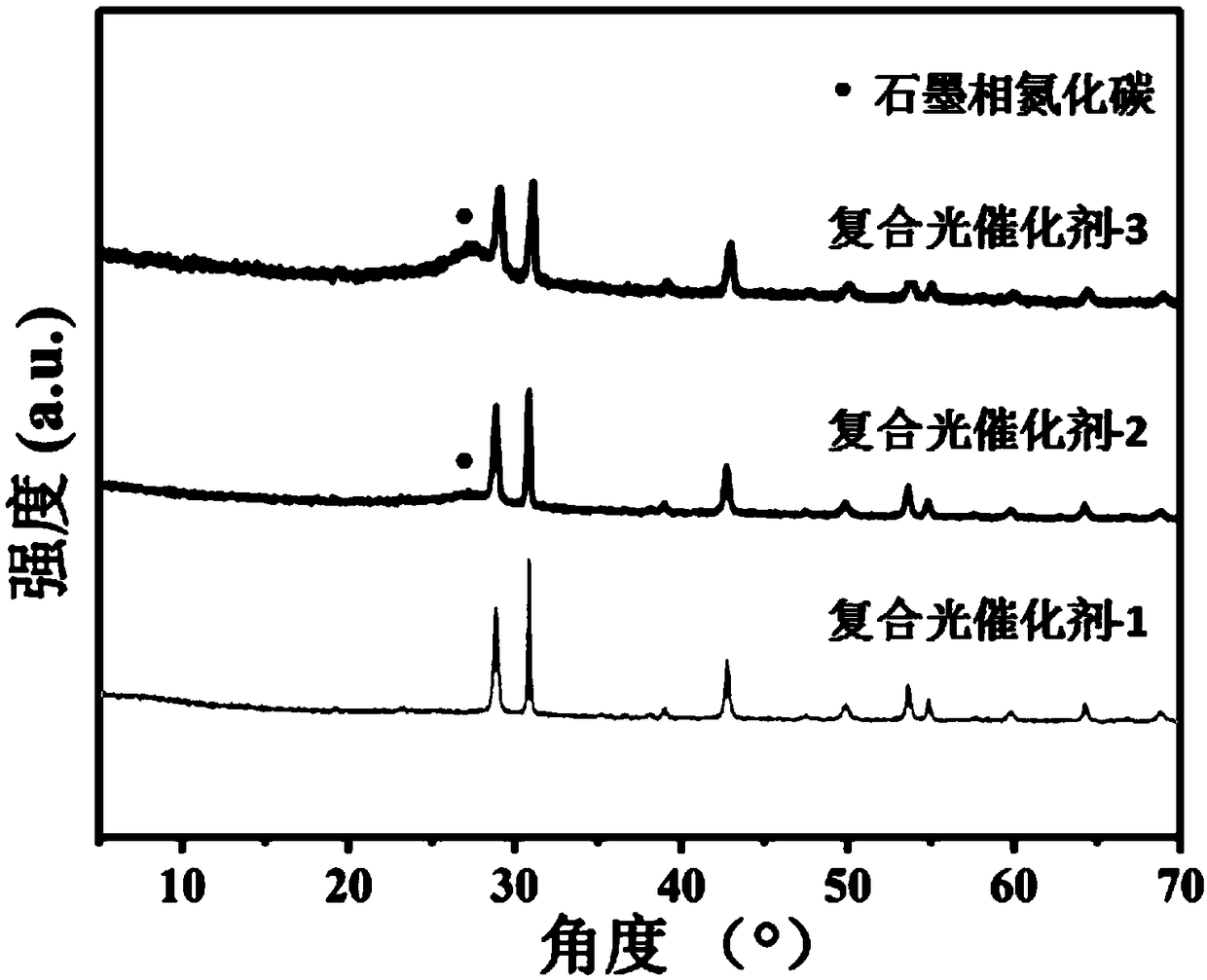
[0046] Step A4: Washing and drying the light yellow precipitate in sequence to obtain a graphite phase carbon nitride-barium niobate composite photocatalyst.
Embodiment 3
[0047] Example 3 of the present invention is a method for preparing a composite photocatalyst. Based on Example 2, the mass ratio of the barium niobate nanosheets to the urea is 1:20.
[0048] Example 4 of the present invention is a method for preparing a composite photocatalyst. On the basis of Example 2 or 3, the heating is performed by a water bath method, and the water bath temperature of the water bath method is 80°C; the calcination is performed in a Maffei furnace It was carried out in medium and lasted at 500°C for 2 hours.
[0049] Example 5 of the present invention is a method for preparing a composite photocatalyst. On the basis of any of Examples 2 to 4, the barium niobate nanosheets are prepared by the following steps:
[0050] Step B1, dissolving barium hydroxide in the first ultrapure water and fully stirring to obtain solution A; dissolving niobium pentachloride in absolute ethanol and fully stirring to obtain solution B;
[0051] Step B2, adding the solution B to the ...
Embodiment 6
[0054] Example 6 of the present invention is a method for preparing a composite photocatalyst. On the basis of Example 5, the barium hydroxide is barium hydroxide octahydrate.
[0055] Example 7 of the present invention is a method for preparing a composite photocatalyst. On the basis of Example 6, the mass ratio of the barium hydroxide octahydrate to the niobium pentachloride is 5.85:1, and the first ultrapure The volume ratio of water to the absolute ethanol is 5:1.
[0056] Example 8 of the present invention is a method for preparing a composite photocatalyst. On the basis of any one of Examples 5 to 7, the hydrothermal reaction lasts for 48 hours at 180°C.
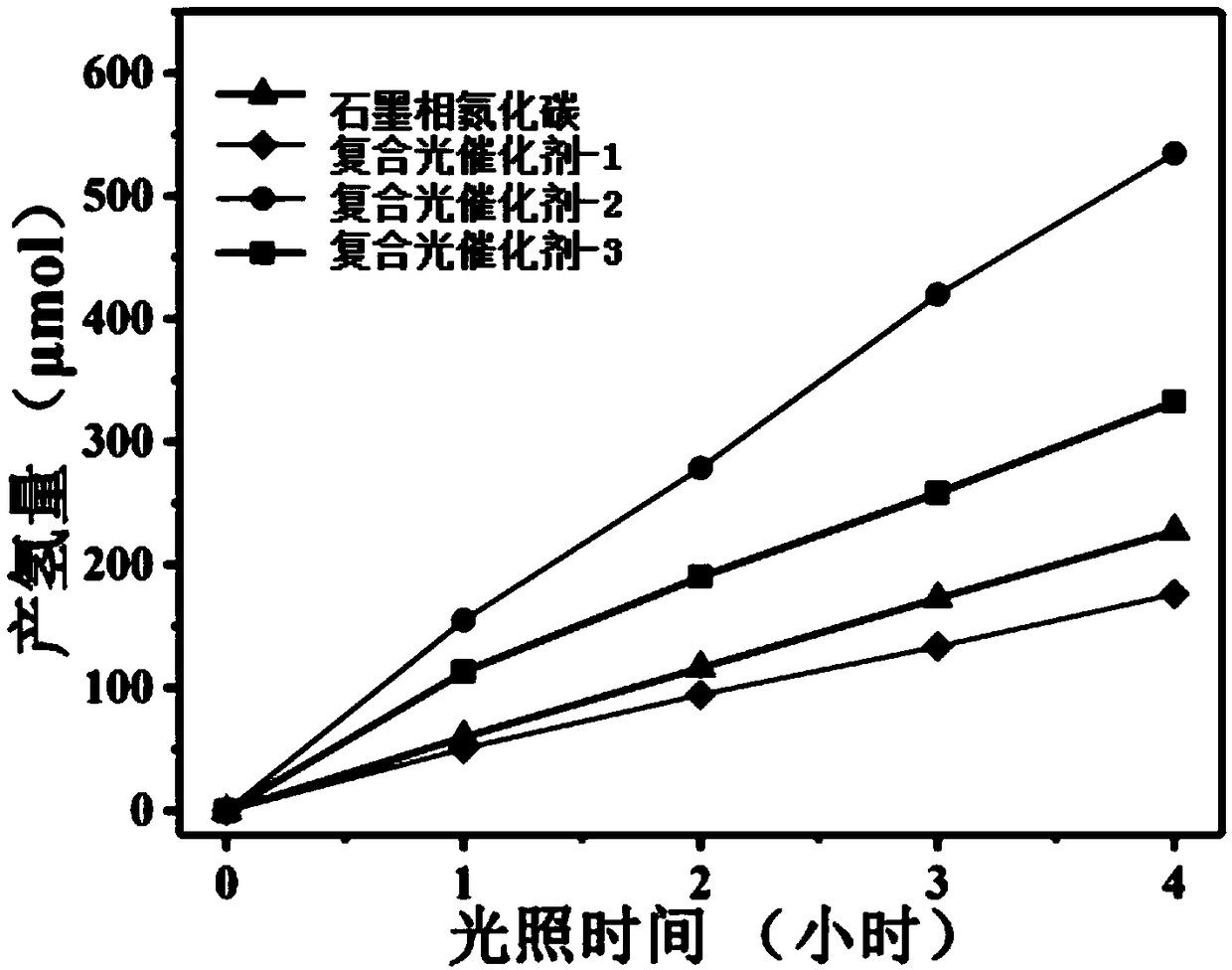
[0057] Embodiment 9 of the present invention is a method for photocatalytic hydrogen production of a composite photocatalyst, which uses the graphite phase carbon nitride-barium niobate composite photocatalyst for photocatalytic hydrogen production.
[0058] Embodiment 10 of the present invention is a method for photocatalytic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com