Bimetallic nitride Co3W3N, preparation method and application thereof
A nitride and bimetal technology, applied in the field of bimetallic nitride Co3W3N and its preparation, can solve problems such as poor catalytic activity, and achieve the effects of poor cycle stability, high nitrogen reduction catalytic activity and large yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Prepare tungsten hexachloride and cobalt chloride in equimolar proportions respectively to make tungsten hexachloride aqueous solution and cobalt chloride aqueous solution, and then slowly add the cobalt chloride aqueous solution to the tungsten hexachloride aqueous solution, and fully stir for 2 hours , making a mixed solution; wherein, the concentration of tungsten hexachloride in the tungsten hexachloride aqueous solution is 1mol / L, and the concentration of cobalt chloride in the cobalt chloride aqueous solution is 1mol / L;
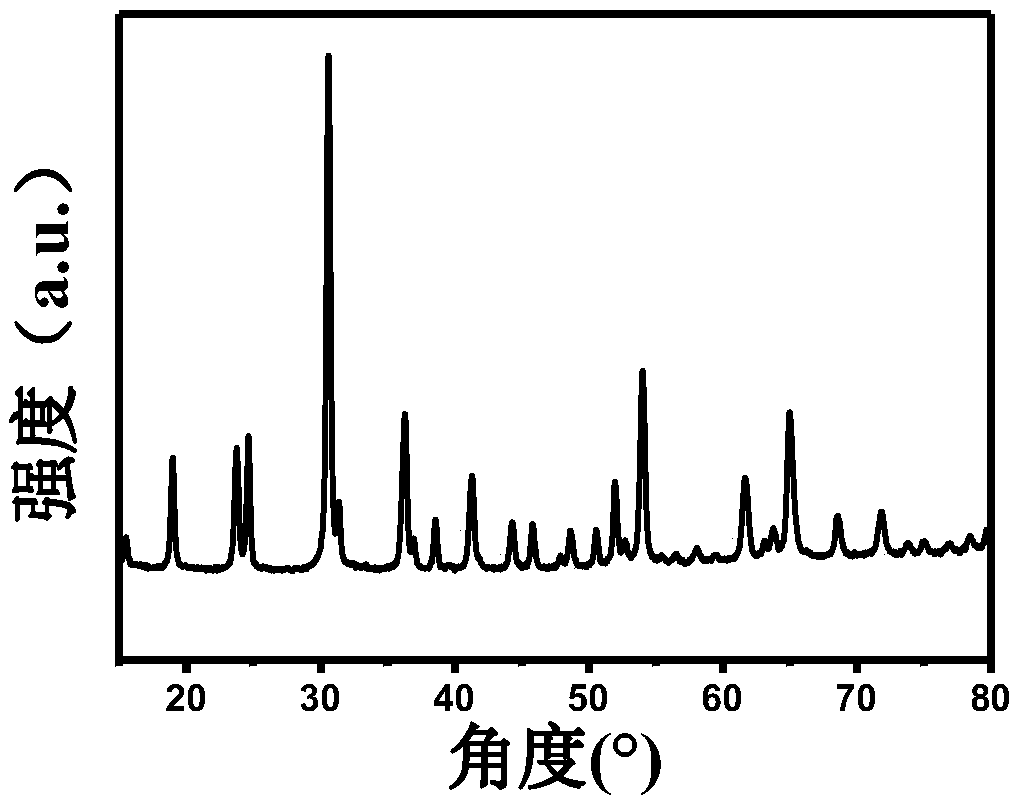
[0028] (2) Transfer the above mixed solution to the reaction kettle, after hydrothermal reaction at 120°C for 24 hours, wash and filter with water, evaporate to dryness at room temperature, and obtain the double transition metal oxide CoWO 4 , followed by the CoWO 4 Preheat treatment at 150°C and air atmosphere for 2 hours;
[0029] (3) CoWO after the above preheating treatment 4 , placed in an ammonia atmosphere tube furnace, heated up to ...
Embodiment 2
[0032] (1) Prepare tungsten hexachloride and cobalt sulfate in equimolar proportions respectively to make tungsten hexachloride aqueous solution and cobalt sulfate aqueous solution, and then slowly add cobalt sulfate aqueous solution into tungsten hexachloride aqueous solution, and fully stir for 2 hours to obtain Mixed solution; wherein, the concentration of tungsten hexachloride in the tungsten hexachloride aqueous solution is 0.5mol / L, and the concentration of cobalt sulfate in the cobalt sulfate aqueous solution is 0.5mol / L;
[0033] (2) Transfer the above mixed solution to a reaction kettle, and after hydrothermal reaction at 160°C for 12 hours, wash and filter with water, and evaporate to dryness at room temperature to obtain the double transition metal oxide CoWO 4 , followed by the CoWO 4 Preheat treatment at 160°C and air atmosphere for 2 hours;
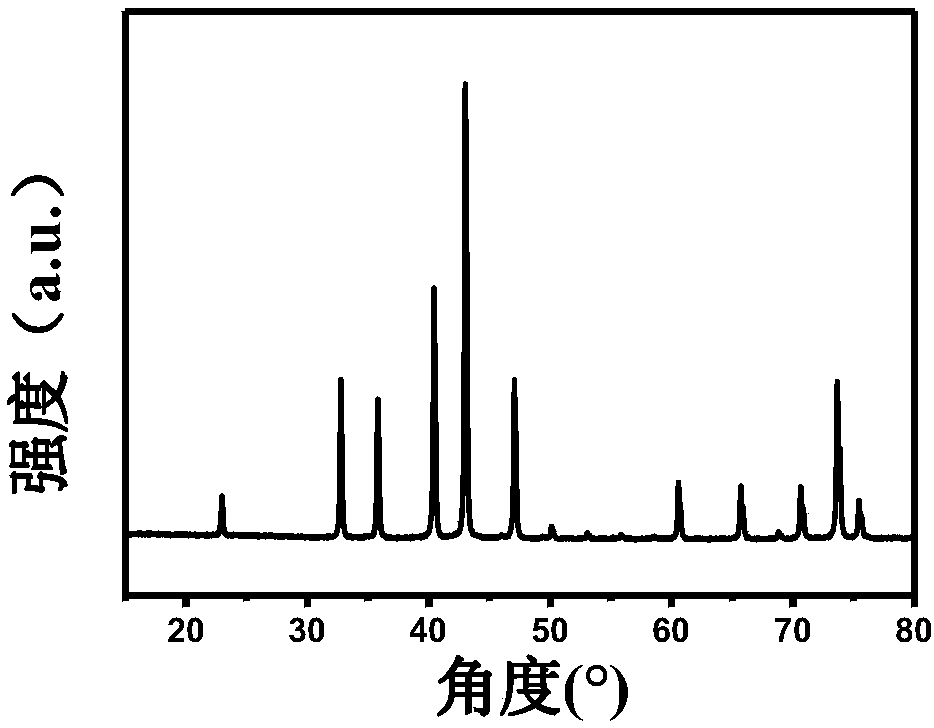
[0034] (3) CoWO after the above preheating treatment 4 , placed in an ammonia atmosphere tube furnace, heated up to 650°...
Embodiment 3
[0036](1) Sodium tungstate and cobalt acetylacetonate in equimolar proportions were prepared respectively into sodium tungstate aqueous solution and cobalt acetylacetonate aqueous solution, and then the cobalt acetylacetonate aqueous solution was slowly added dropwise to the sodium tungstate aqueous solution, and fully stirred for 2 hours to obtain Mixed solution; wherein, the concentration of sodium tungstate in the sodium tungstate solution is 1.5mol / L, and the concentration of cobalt acetylacetonate in the cobalt acetylacetonate aqueous solution is 1.5mol / L;
[0037] (2) Transfer the above mixed solution to the reaction kettle, and after hydrothermal reaction at 140°C for 16 hours, wash and filter with water, evaporate to dryness at room temperature, and obtain the double transition metal oxide CoWO 4 , followed by the CoWO 4 Preheat treatment at 180°C for 2 hours in air atmosphere;
[0038] (3) CoWO after the above preheating treatment 4 , placed in an ammonia atmosphere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com