N-type conjugated polymer containing oligomeric ethylene glycol side chain modified naphthalene diimide unit and application of conjugated polymer in organic photoelectric devices
A technology of oligoethylene glycol and naphthalene diimide, which is applied in the field of n-type conjugated polymers, can solve the problems of poor stability and difficulty in controlling the morphology of the blended film of the photoactive layer, and achieve excellent stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
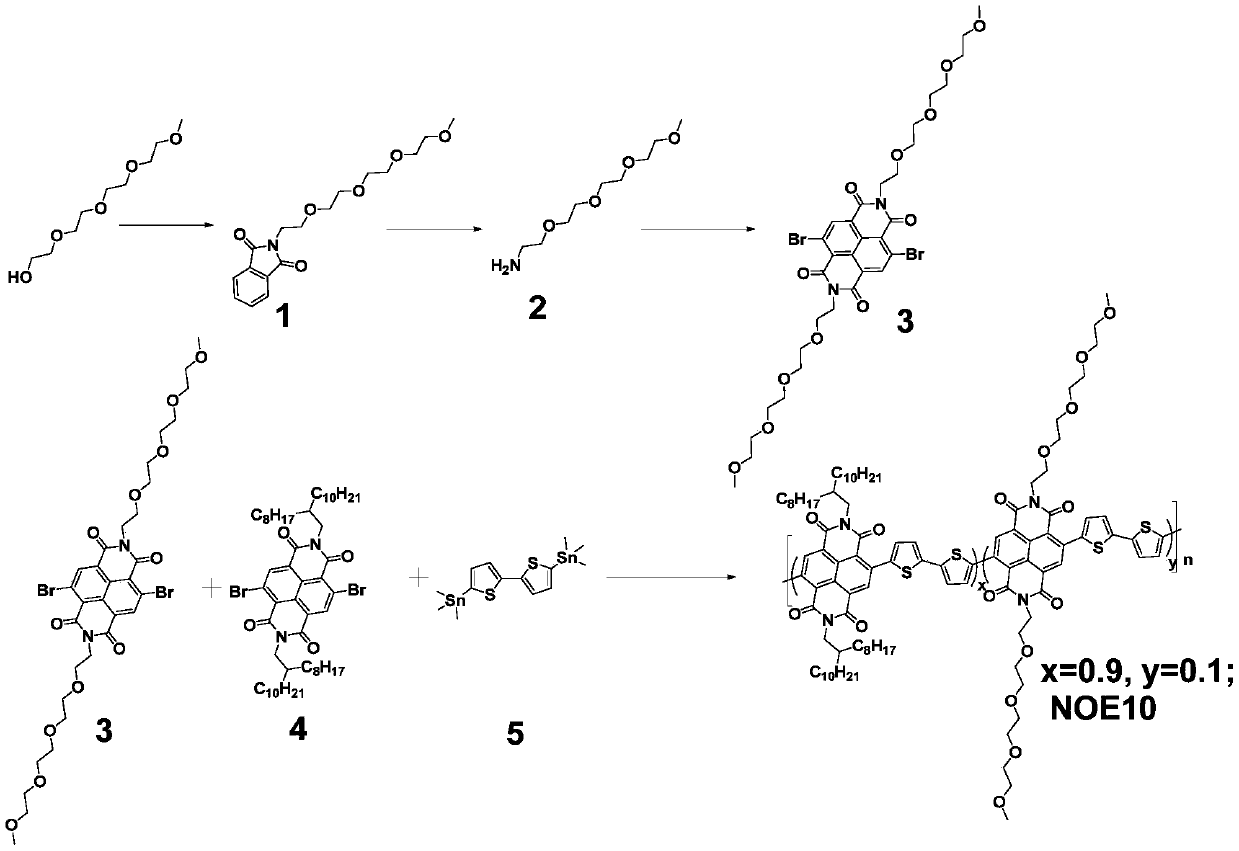
[0027] Preparation of Naphthalimide Monomer (Structure 3) Modified with Oligoethylene Glycol Side Chain.
[0028] The chemical reaction process is as follows, and the specific reaction steps and reaction conditions are as follows:
[0029]
[0030] (1) Raw materials or intermediate reactants, tetraethylene glycol monomethyl ether, diisopropyl azodicarboxylate, phthalimide, triphenylphosphine, hydrazine hydrate, 2,6-dibromonaphthalene-1 ,4,5,8-Tetracarboxylic dianhydride is used directly as a commercial product.
[0031] (2) Preparation of intermediate 1
[0032] Under nitrogen protection, tetraethylene glycol monomethyl ether (6g), phthalimide (4.5g) and triphenylphosphine (8.5g) were dissolved in 150 ml of dichloromethane, at 0 ° C 6.5 g of diisopropyl azodicarboxylate was slowly added dropwise to the reaction liquid, and stirred at room temperature for 12 hours. After the reaction was completed, the solvent was removed, and purified by silica gel column to obtain 7 g o...
Embodiment 2
[0038] Preparation of n-Type Conjugated Polymer (NOE10) Containing Naphthalimide Units Modified by Oligoethylene Glycol Side Chains
[0039] The chemical reaction process is as follows, and the specific reaction steps and reaction conditions are as follows:
[0040]
[0041] (1) Raw materials or intermediate reactants, monomer 4, monomer 5, and tetrakistriphenylphosphine palladium are used directly as commercial products.
[0042] (2) Under nitrogen protection, dissolve monomer 3 (0.02 mmol), monomer 4 (0.18 mmol), monomer 5 (0.2 mmol) and 3 mg of tetraphenylphosphine palladium in 2 ml of chlorine In benzene, stir at 120°C for 12 hours. After the reaction, the polymer was precipitated in methanol, extracted with methanol, acetone, and n-hexane, and finally extracted with chloroform to obtain the final polymer. The final polymer NOE10 was obtained after precipitation with methanol again, 180 mg, and the yield was 90%.
Embodiment 3
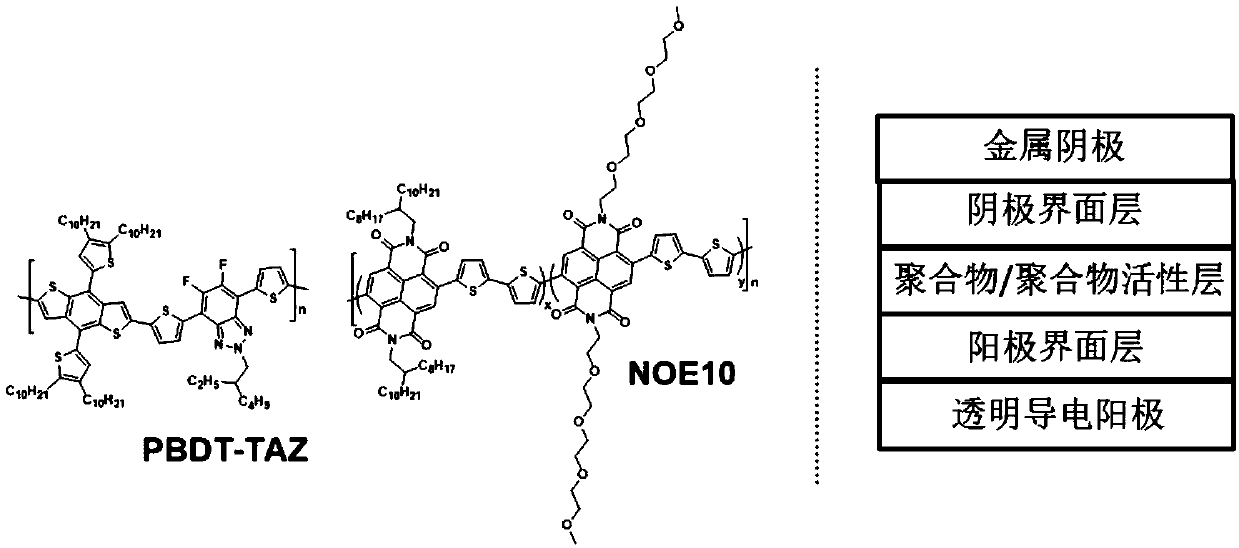
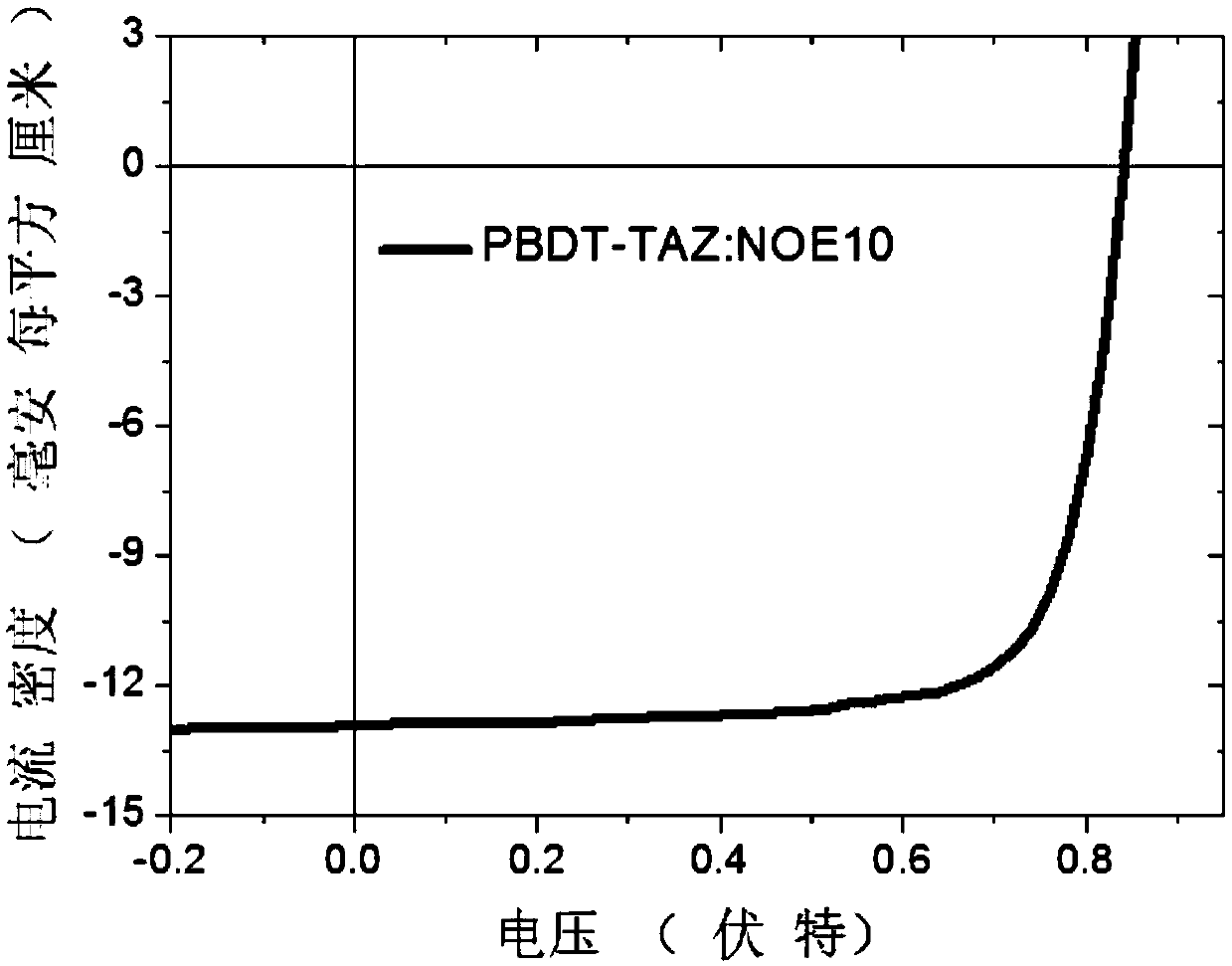
[0044] The polymer material obtained in Example 2 is taken as an example to illustrate the application of this polymer material as a polymer acceptor in polymer / polymer solar cell devices.
[0045] The following examples will illustrate the n-type conjugated polymer containing oligoethylene glycol side chain modified naphthalene diimide units proposed by the present invention and its application process in organic photoelectric devices, but the present invention does not limited to the examples given.
[0046] The specific preparation process of the device is as follows:
[0047]Spin-coat a layer of 40nm PEDOT:PSS hole transport layer on ITO, then spin-coat the photoactive layer of polymer donor PBDT-TAZ and NOE10 blended with about 100nm, and then spin-coat about 5nm of amine-based polymer The quaternary ammonium bromide salt of fluorene (PFN-Br) was used as the cathode interface layer, and then a 100 nanometer Ag layer was evaporated to complete the preparation of the devic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com