A novel alginate lyase aly08 and its application
A technology of alginate lyase and molecular alginate, applied in the directions of lyase, carbon-oxygen lyase, application, etc., can solve the problems of poor thermal stability and thermal recovery, unable to achieve industrial application, easy inactivation, etc., and achieve high activity , Good industrial application prospects, simple purification method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
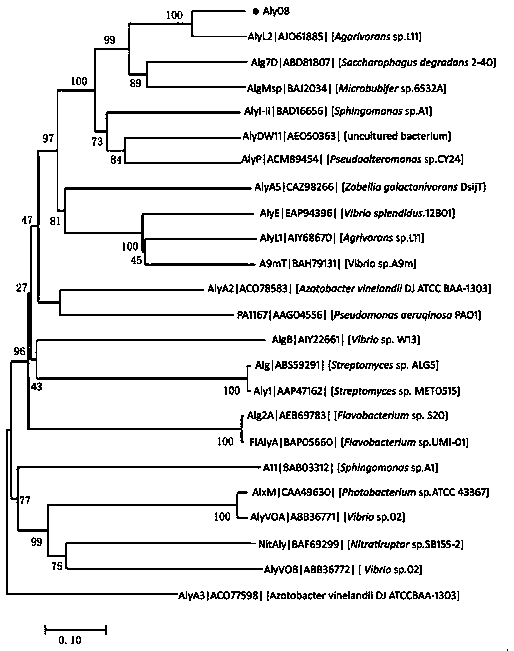
[0033] Example 1 Sequence analysis of alginate lyase Aly08
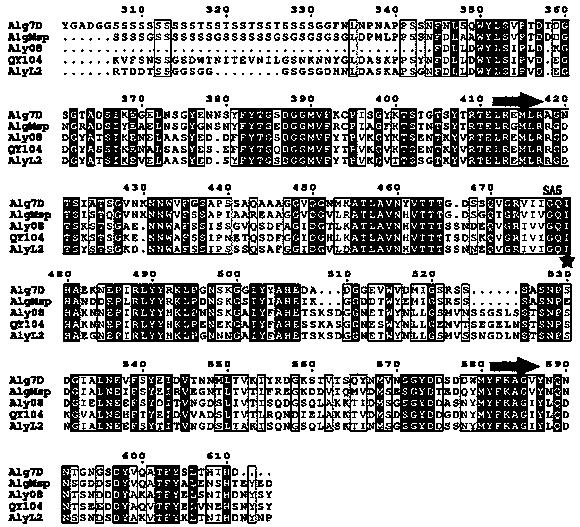
[0034] The enzyme-producing gene aly08 of alginate lyase Aly08 in the present invention is a fully synthetic sequence (synthesized by Huada Gene Company), which contains 897 base sequences and encodes 299 amino acid sequences. Using the conserved domain analysis of the National Center for Biotechnology Information (NCBI) to analyze the Conserved domain (CDD) and the multiple sequence alignment Basic Local Alignment Search Tool (Blast), it was found that the sequence contained a second superfamily of alginate lyase (Alginate lyase 2 superfamily) conserved region. Among the reported alginate lyases, the one with the highest amino acid sequence similarity to Aly08 is the alginate lyase AlyL2 (Genbank AJO61885) of the polysaccharide lyase family 7 (PL-7). The amino acid sequence similarity between the two ( Identity) is 78%.
[0035] The amino acid sequence of the alginate lyase Aly08 described in the present invention...
Embodiment 2
[0037] Example 2 Recombinant expression of alginate lyase Aly08
[0038] The fully synthesized algA1 gene sequence in Example 1 uses restriction enzymes Nco I and Xho I as restriction sites, and the recombinant primers are designed as follows (underlined restriction endonuclease sites, italics restriction endonucleases protected bases):
[0039] Forward primer: SEQ ID NO.3: Paly08F:
[0040] 5'-CATG CCATGG GTATGTTATTGAACAAAAT-3’ (Nco I)
[0041] Reverse primer: SEQ ID NO.4: Paly08R:
[0042] 5'-CCG CTCGAG GTAAGAGTAGTTGTC -3' (Xho I)
[0043] PCR amplification conditions were: pre-denaturation at 94°C for 3 minutes; denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 1 minute, a total of 30 cycles; extension at 72°C for 5 minutes; stabilization at 4°C for 15 minutes. The DNA polymerase used in the PCR reaction was PrimerstarHS purchased from Dalian Bao Biological Company.
[0044] The PCR product was double-digested with restric...
Embodiment 3
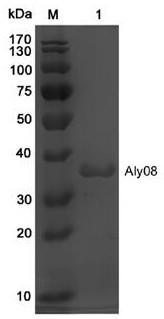
[0046] Example 3 Fermentation and purification preparation method of alginate lyase Aly08
[0047] The Escherichia coli BL21(DE3) / pET22b-Aly08 constructed in Example 2 was transferred to LB liquid medium (50 μg / mL ampicillin), and cultured in a shaker at 37°C at 180 rpm to OD 600 =0.6. The 5 L fermenter was loaded with 60% (3 L) Terrific Broth (TB) medium, and sterilized in advance; 50 μg / mL ampicillin was added to the fermenter, and the cultured bacteria in the Erlenmeyer flask were The solution was inoculated into a 5 L fermenter according to the inoculum amount of 5%. Adjust the initial ventilation rate to 60L / h, the initial rotation speed to 300 rpm, the temperature at 37°C, and the dissolved oxygen at 10-60%; when the bacteria grow to OD 600 = 5.0, add the inducer isopropyl-β-D-thiogalactoside (IPTG) at a final concentration of 0.1 mM, and induce at 20°C for 60 h. Feed was carried out during this period. Feed medium (300 g glycerol dissolved in 100 mM pH7.6 phosphate bu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com