An in situ injectable temperature-sensitive response water-soluble chitosan composite hydrogel for lacrimal embolism, its preparation method and application
A technology of water-soluble chitosan and composite hydrogel, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc. Reduce the patient's compliance with treatment, increase the patient's economic burden, etc., to achieve the effect of low operation cost, low toxicity and side effects, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
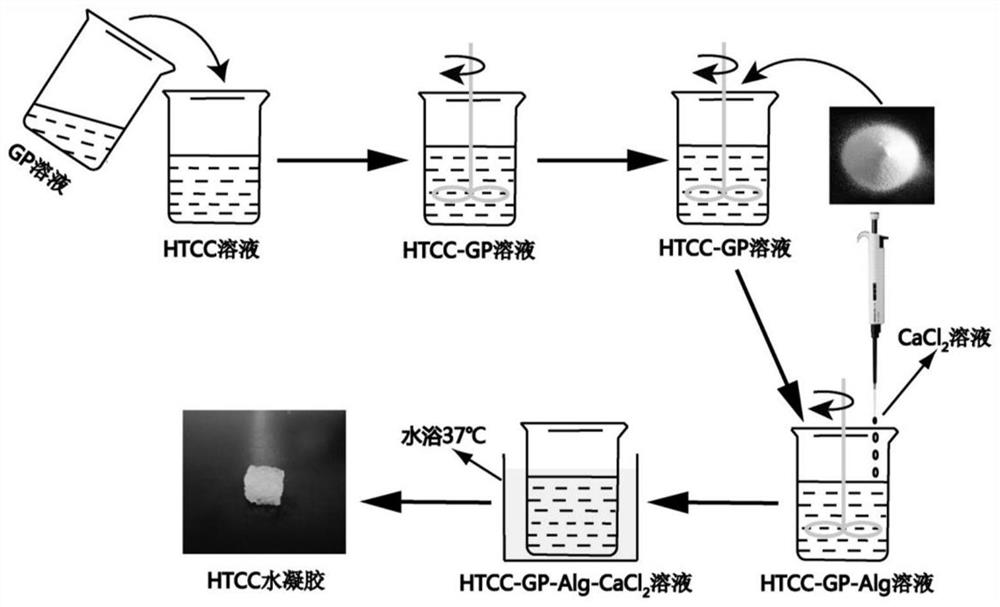
[0044]Seefigure 1 , Is the experimental process of the present invention, the specific method is as follows: accurately weigh 6wt% of HTCC powder and dissolve it in deionized water to prepare an HTCC) aqueous solution, similarly prepare 14wt% of β-glycerophosphate sodium (GP) aqueous solution, and adjust the volume The HTCC aqueous solution and the GP aqueous solution with a ratio of 1:0.7 are mixed and stirred uniformly, and then 2% Na-Alg powder is added to the above-mentioned HTCC-GP mixed aqueous solution, and fully stirred and dissolved. Add 3% CaCl to the HTCC-GP-Na-Alg mixed aqueous solution of Example 12The aqueous solution is stirred evenly and moved into the mold, and the mold is placed in a constant temperature water bath at 37°C to make it gelatinize and transform into a hydrogel.
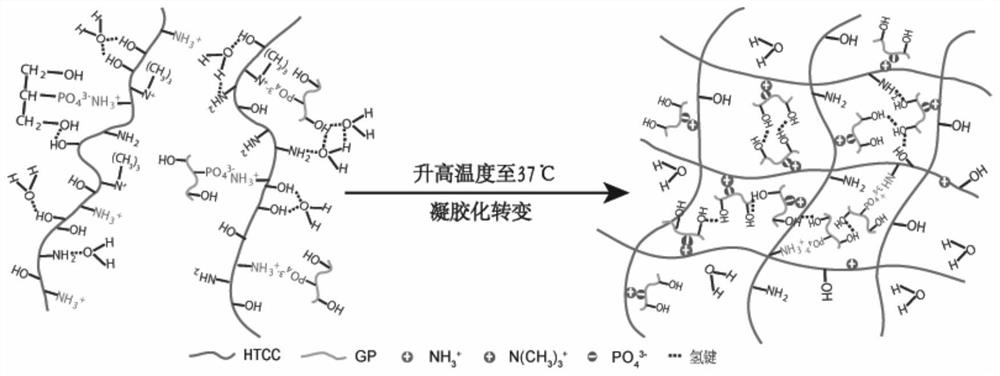
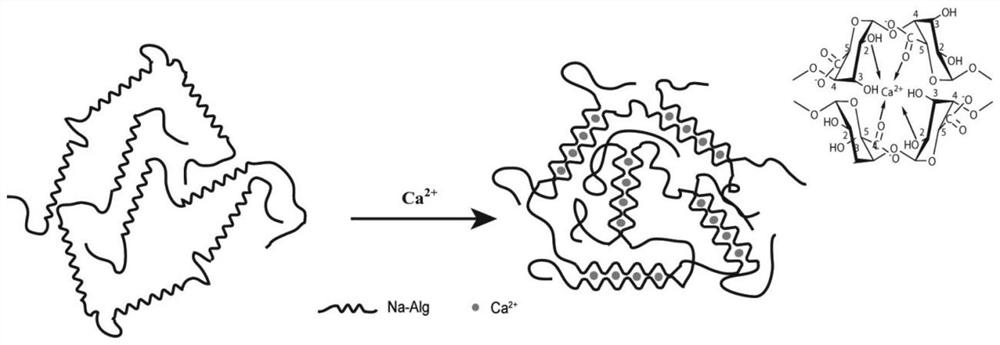
[0045]The mechanism in the gelation process is asfigure 2 ,image 3 andFigure 4 ,figure 2 It is a diagram of the gelation mechanism of HTCC-GP. At room temperature, water molecules bind to the lo...
Embodiment 2
[0048]The HTCC-GP-Na-Alg-Ca of Example 12+The mixed aqueous solution was transferred into the vial, the result was fromFigure 5 It can be seen that it is a fluid mixed solution at room temperature. After gelation at 37°C, an in-situ injectable temperature-sensitive chitosan quaternary ammonium salt complex water for lacrimal embolism is obtained that loses fluidity. gel(Figure 6 ).
Embodiment 3
[0050]Dissolve 1wt%, 3wt%, 5wt%, 7wt% and 9wt% of HTCC powder in deionized water to prepare an HTCC aqueous solution. Similarly, prepare a 15% GP aqueous solution with a volume ratio of 1:0.8 to prepare HTCC-GP Mix the aqueous solution, move it into the mold, and undergo gelation at 37°C to obtain an in-situ injectable temperature-sensitive chitosan quaternary ammonium salt gel for lacrimal embolization, and pass the test tube inversion method in Example 1 The system loses fluidity to observe its gelation time. The specific implementation method is: place the parallel vial with the same formula at a constant temperature of 37℃, and use a stopwatch to tilt the vial every 10s to observe the system If the system loses fluidity or the liquid level does not change after tilting, this time is recorded as the phase inversion time of the gel. Observe the influence of HTCC concentration changes on the gelation time (Figure 7 ),FromFigure 7 It can be seen that the gelation time first shortens...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com