Chalcone compound derived from Beta-ionone and preparation method and application thereof
A technology of ionone and chalcone, which is applied in the field of chalcones derived from β-ionone and its preparation, can solve the report on the application of the preparation method of β-ionone-chalcones to control pests that has not been seen and other problems, to achieve the effect of direct control, less by-products and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
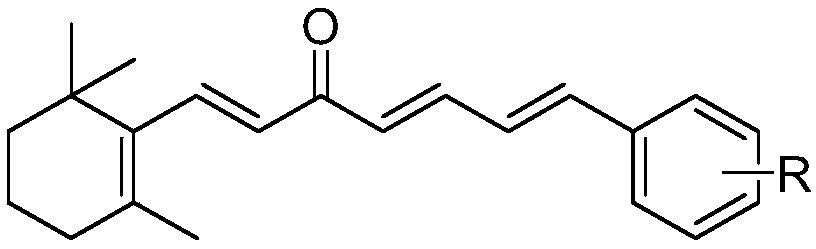
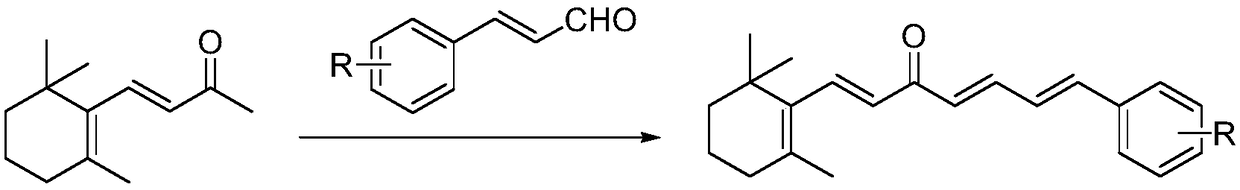
[0034] The embodiment of the present invention provides a preparation method of chalcones derived from β-ionone, specifically as follows:
[0035] Dissolve 4.6mmol of β-ionone and 6.0mmol of substituted cinnamaldehyde in 10mL of ethanol, respectively, and add them to a 50mL three-neck flask, slowly add 15mL of 1mmol / mL sodium hydroxide aqueous solution to the three-necked flask, and stir at room temperature to react. Analyze and monitor reactions.
[0036] After the reaction was complete, the pH was adjusted to 6-7 with 10% hydrochloric acid solution, followed by extraction with diethyl ether twice (50 mL of diethyl ether each time), and the organic phase was dried and concentrated.
[0037] The target compound was obtained by column chromatography using petroleum ether and ethyl acetate as eluents.
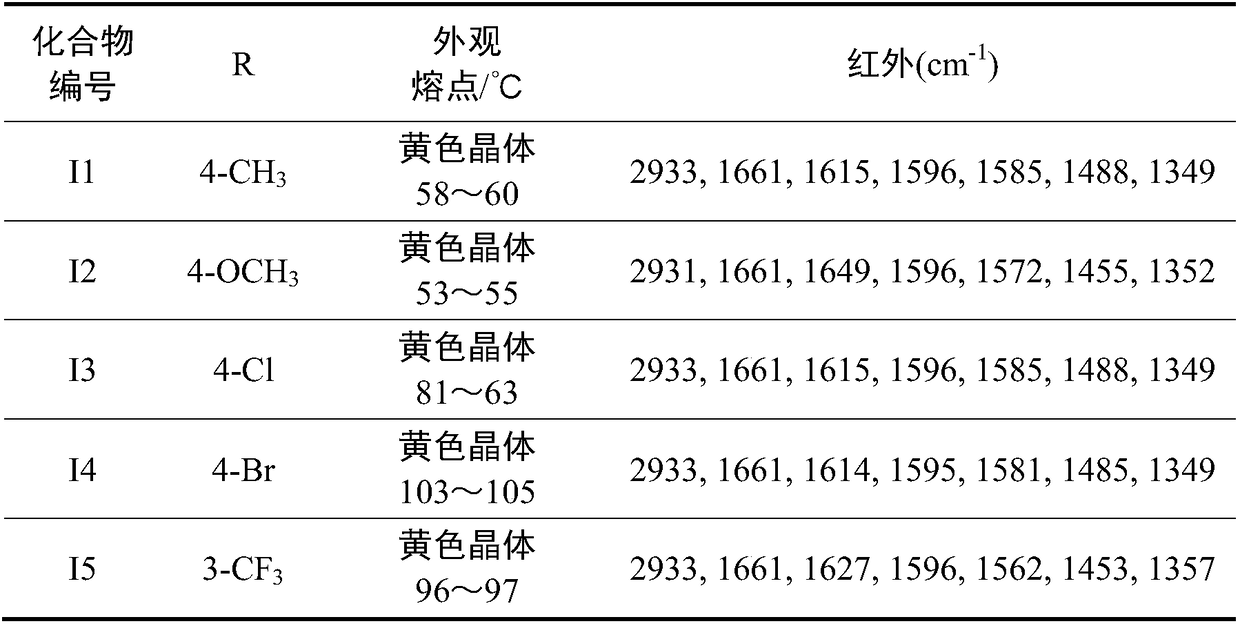
[0038] The structure, physical and chemical parameters and infrared data of the obtained product are shown in Table 1.
[0039] The H NMR spectrum and high resolution mass spec...
experiment example 1
[0045] Experimental Example 1 Determination of the Attractive Activity of Chalcones Derived from β-Ionone to Lygus Chrysophylla
[0046] Dissolve the product prepared in the embodiment of the present invention in dichloromethane, take the solution and add it to rubber lures (each rubber lure contains 10 mg of medicine), and after the dichloromethane solvent has evaporated, add clean dichloromethane , so that the chalcones derived from β-ionone fully penetrate into the rubber; after the dichloromethane solvent evaporates again, wrap the lure with tinfoil, put it in a ziplock bag and place it in a -4°C refrigerator for later use.
[0047] In the alfalfa field, the trapping test of Lygus spp. was carried out, and the specific operation was as follows: select the alfalfa field with the same growth, consistent management conditions, and large and neat plots as the experimental field, and fix the above-mentioned prepared lure on the boat-type trap (at the same time, use CK and β-ion...
experiment example 2
[0052] Experimental example 2 Determination of the insecticidal activity of chalcones derived from β-ionone against diamondback moth and Tetranychus cinnabarinus
[0053]Dissolve the product (3 mg) obtained in the example of the present invention in a mixed solvent (2.5 mL) of acetone / methanol (1:1), add 2.5 mL of standing tap water containing 2‰ Tween 80, and stir to obtain 600 mg / L of the solution to be tested.
[0054] A 10 mg / L imidacloprid solution was used as a positive control, and acetone / methanol / water (1:1:2, containing 1‰ Tween 80) was used as a blank control.
[0055] According to the standard operating procedure of insecticide micro-screening, the Airbrush spray method was used. The sprayer was a VL-type airbrush produced by Paasche Airbrush Company in the United States, and the spray pressure was 10psi (approximately 0.7kg / cm2). 2 ), the spray volume is 0.5mL, and the spray distance is 15-20cm.
[0056] See Table 4 for test targets (Plutella xylostella xyloste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com