Antibody, polypeptide and nucleic acid combined treatment targeting carrier as well as preparation method and application thereof
A therapeutic target and carrier technology, applied in the direction of antibody medical components, antibodies, drug combinations, etc., can solve the problems of high toxicity, difficult to safely and effectively inhibit tumor growth and metastasis, and achieve the effect of preventing leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
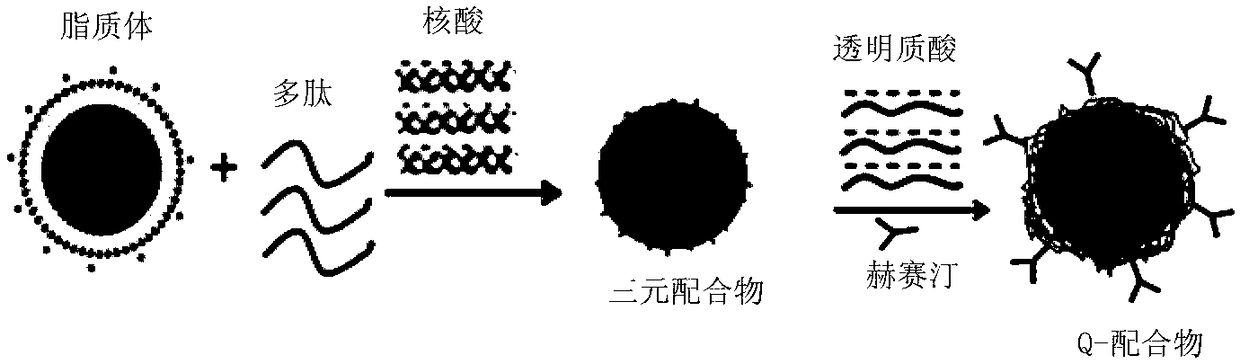
[0049] The present invention relates to a method for preparing the above-mentioned antibody, polypeptide and nucleic acid combination therapy targeting carrier, comprising the following steps:
[0050] a. Connection reaction between Herceptin and hyaluronic acid (HA)
[0051] Herceptin is a commercially available human injection, and the excipients in it are removed by ultrafiltration in a 100k ultrafiltration centrifuge tube: L-histidine hydrochloride and L-histidine, because histidine is also a basic amino acid. The response is disturbed. The concentration after ultrafiltration is 17.6mg / ml (9.51×10 -5 mol / L). Each molecule of Herceptin contains 44 histidines. The specific experimental steps are as follows: activation of carboxyl groups, pH 6.0 MES buffer solution, HA, N-hydroxysuccinimide (NHS), + 1-(3-dimethylaminopropyl)-3-ethylcarbodi The molar concentrations of imine hydrochloride (EDC) were 0.2mM, 5mM, and 2mM respectively, and the reaction was activated at room te...
Embodiment 1
[0055] Example 1, Preparation method of antibody, polypeptide and nucleic acid combination therapy targeting carrier
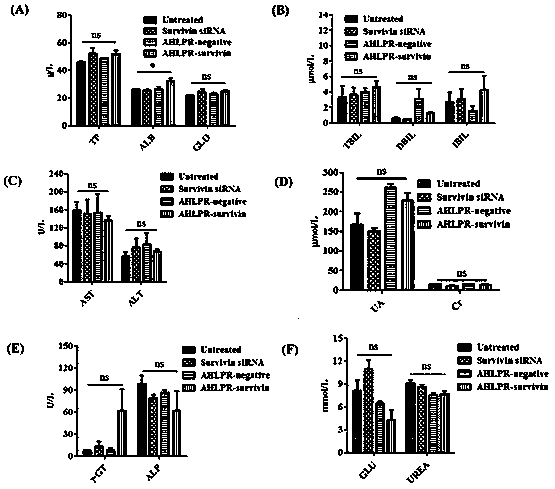
[0056] Such as figure 1As shown, the system for measuring the physicochemical properties of antibody, polypeptide and nucleic acid combination therapy targeting carrier is carried out in aqueous solution. After mixing the cationic liposomes and polypeptides in different mass ratios, they are added into the nucleic acid aqueous solution and continue to mix evenly. In the specific experiment, the mass ratios of cationic liposomes, polypeptides and nucleic acids were 1:8:1, 1:4:1 and 1:2:1, respectively, and incubated at room temperature for 30 minutes, and then different proportions of HA- HER was added to the mixture and mixed well, and the mass ratio of HA to DNA or siRNA in HA-HER was 10:1:8:1, 14:1:4:1 and 30:1:2:1 respectively. Set aside for 10-25 minutes. For the carrier system used in cell experiments, serum-free medium can be used instead of ultrapure...
Embodiment 2
[0057] Example 2, Zeta Potential of Antibody, Polypeptide and Nucleic Acid Combination Therapy Targeting Carrier
[0058] Zetasizer 2000 was used to measure the surface charge of the complex, the sample measurement time was set to automatic, each sample was measured 3 times, and the average value was plotted. The results of the zeta potential measurement of the complex are shown in Table 1. It can be seen from Table 1 that when hyaluronic acid is used as the shell shielding charge, the potential potential of nanoparticle HLPR is negative charge -38 mV, and when HA-HER is used as shell shielding charge, the zeta potential of nanoparticle AHLPR is close to neutral charge (-12 mV). On this basis, we selected HA-HER with a mass ratio of hyaluronic acid to siRNA of 14:1 as the shell to assemble nanocarriers for research.
[0059] Table 1
[0060]
[0061] Table 1 shows the particle size and zeta potential of the targeted carrier AHLPR and the corresponding control group HLPR a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com