Sorafenib solid lipid nanoparticles as well as preparation method and application thereof
A sorafenib lipid and a technology for describing sorafenib lipid, which are applied in the field of sorafenib lipid nanoparticles and their preparation, and can solve the problem of affecting the drug efficiency of sorafenib, not proposed, and not found. Patients with advanced hepatocellular carcinoma, etc., to achieve the effect of enhancing photostability and tumor aggregation, improving toxicity and solubility, and improving blood circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Preparation of Sorafenib Lipid Nanoparticles (SILS for short)
[0035] 1. Prepare phospholipid by mixing dipalmitoylphosphatidylcholine DPPC, cholesterol and DSPE-PEG2000.
[0036] 2. Weigh Sorafenib (SF) and phospholipids, add methanol and chloroform (volume ratio 2:1) and shake to dissolve, obtain the organic phase and remove the organic phase by rotary evaporation with a rotary evaporator to obtain phospholipids containing Sorafenib film.
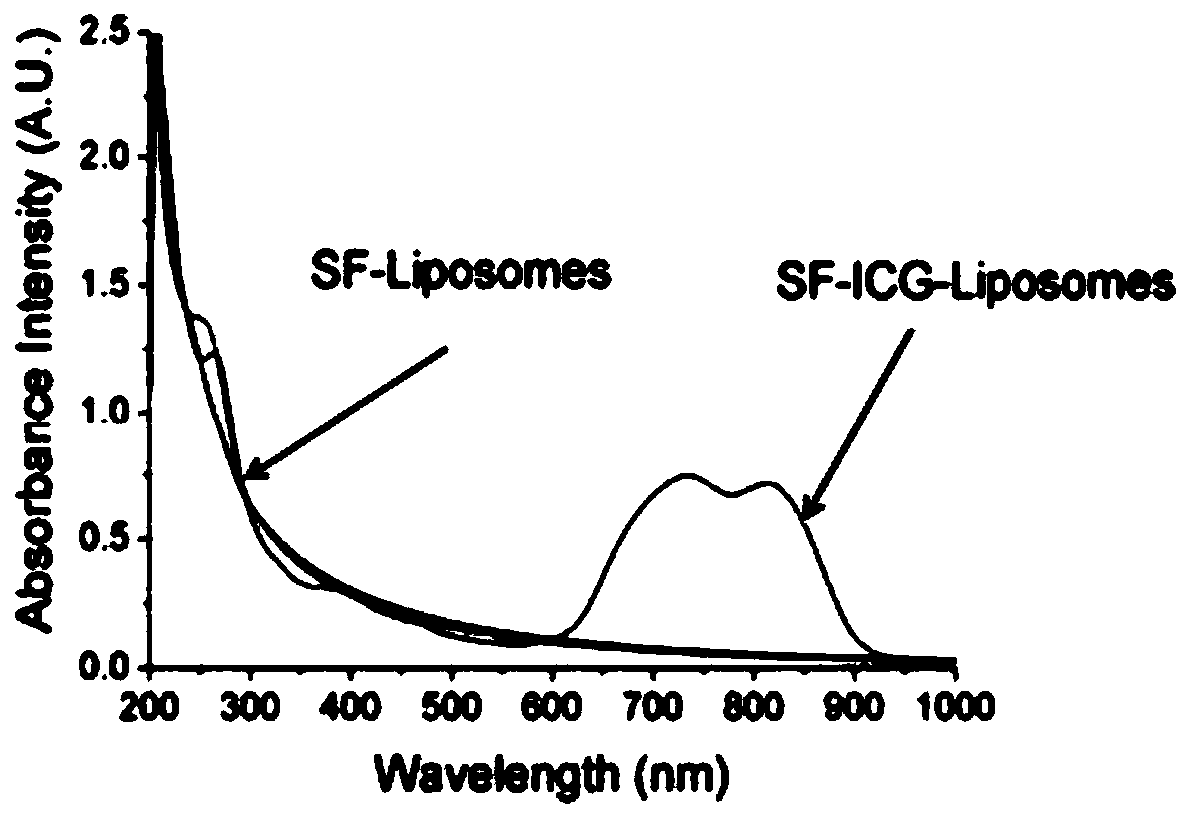
[0037]3. Weigh indocyanine green (ICG), add it into the water for injection, and dissolve to obtain the water phase.
[0038] 4. Keep the temperature at 60°C, add the water phase into the phospholipid film, and stir the mixture with a magnetic stirrer at 320 rpm for 4 hours. Sonicate the prepared liposomes for 20 minutes, extrude once with a 450nm filter membrane, and then repeatedly extrude 5 times through a 200nm and 100nm filter membrane with a liposome extruder, and store at 4°C.
[0039] Simultaneously prepare blank lip...
experiment example 1
[0043] Experimental Example 1 Sorafenib Lipid Nanoparticle Performance Detection
[0044] 1. The morphology of SILS was observed by negative staining method of transmission electron microscope. Such as figure 1 Shown, is the transmission electron microscope figure of this Sorafenib lipid nanoparticle (abbreviation SILS); Transmission electron microscope (TEM) figure has shown clear typical spherical lipid shape, has further confirmed that SILS is stable in water, to The aggregation and fusion of vesicles have a certain inhibitory effect.
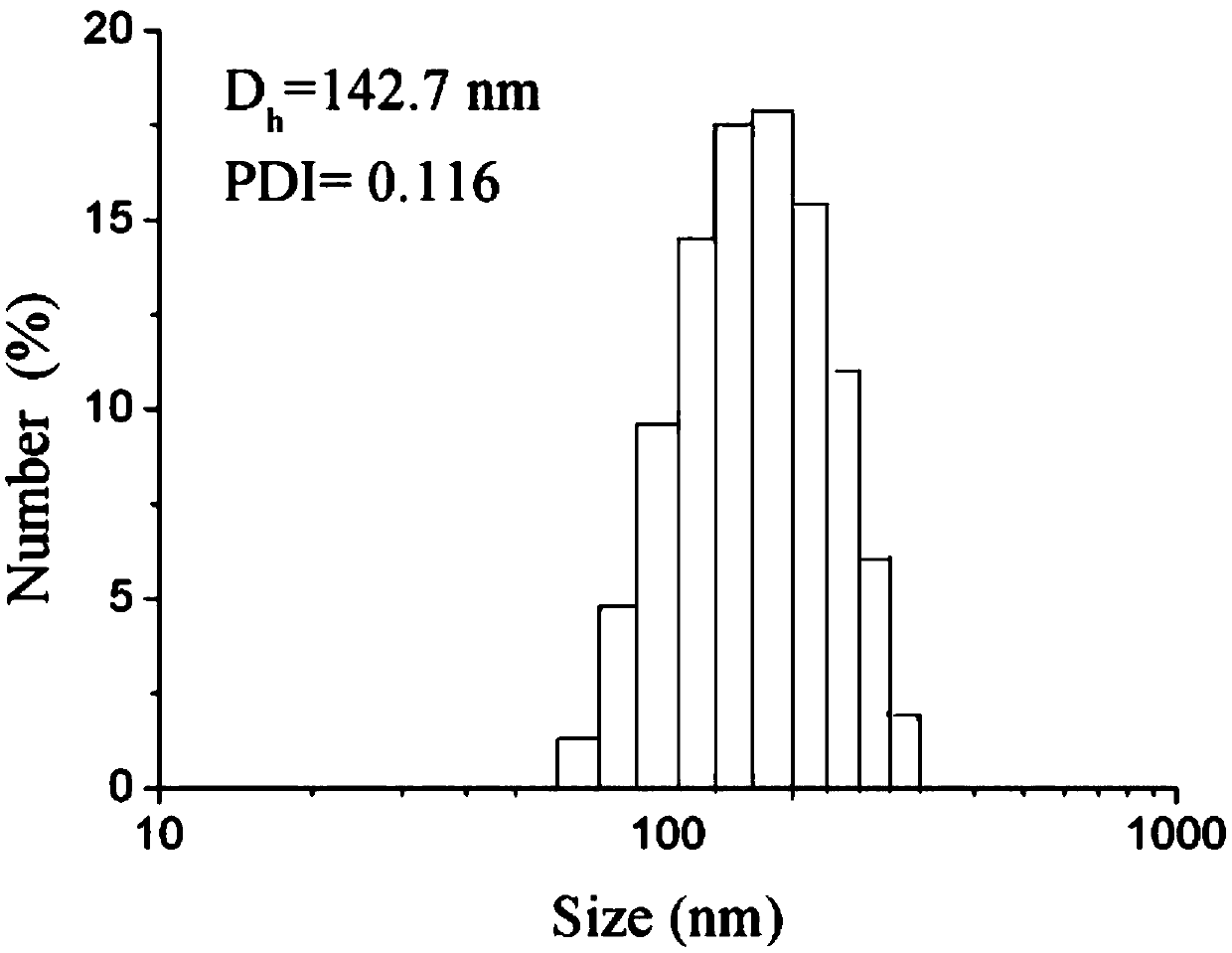
[0045] 2. The particle size, polyhedral index (PDI) and Zeta potential of SILS were further determined by ZetaSizer Nano series nanometer instrument Co., Ltd. Such as figure 2 Shown is the diameter and the polydispersity index (PDI) figure of this Sorafenib lipid nanoparticle; And summarize the following table 1.
[0046] Table 1
[0047] sample
Size (nm)
PDI
SILS
143.4±3.9
0.123±0.008 ...
experiment example 2
[0051] Experimental Example 2 Photothermal Effect of Sorafenib Lipid Nanoparticles
[0052] 1. In order to clarify the photothermal effect of SILS (60μg / mL ICG), we analyzed the near-infrared (808nm, 2W / cm 2 ) temperature rise characteristics of free SF, free ICG and SILS under irradiation, such as Figure 6 shown.
[0053] 2. Thermal image ( Figure 7 ) showed that the maximum temperatures of SILS and free ICG were 65.5°C and 58.9°C, respectively. The increase in the maximum temperature of SILS relative to free ICG may be due to the increased stability and absorbance of ICG by liposomes. Therefore, encapsulated ICG is more effective at increasing temperature than free ICG.
[0054] 3. We also explored the photostability of SILS and free ICG (60μg / mL ICG). First, SILS and free ICG were irradiated with 808nm near-infrared laser (2.0W / cm 2 ) for 3 minutes and then cooled to room temperature. Without near-infrared laser irradiation for 10 min. Typical temperatures in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com