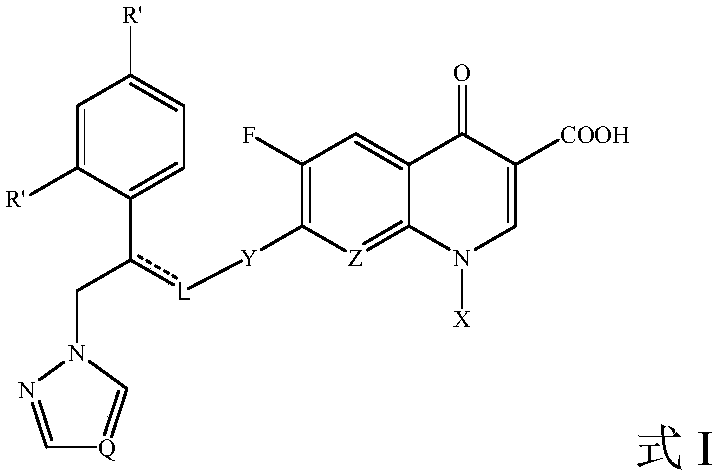
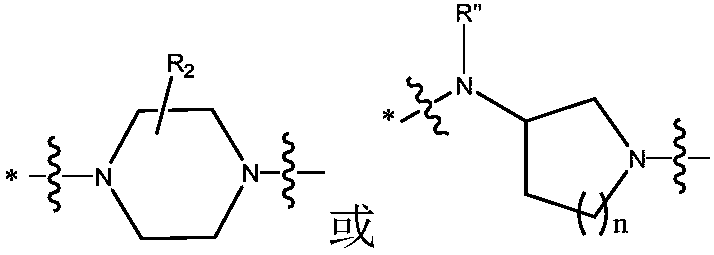
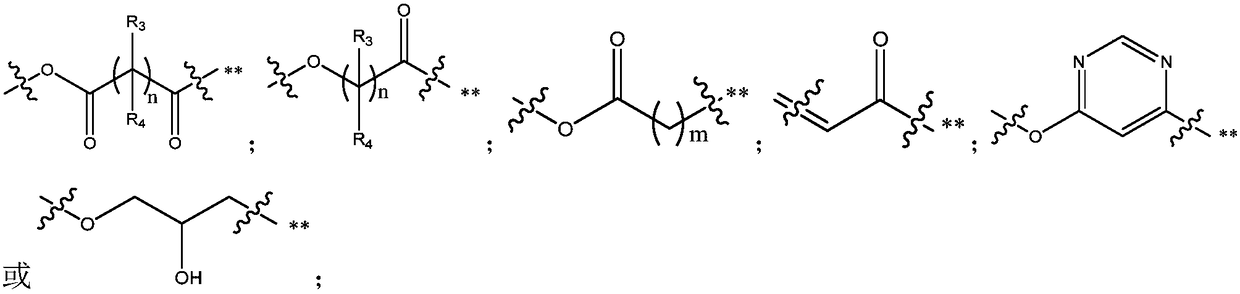
Fluoroquinolone-methyl thiazolyl tetrazolium heterozygous derivative, preparation method and application thereof
A compound, nitrogen oxide technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the synthesis of TM1 series compounds
[0041] The general route is as follows:
[0042]
[0043] Add 2',4'-difluoro-2-[1-(1H-1,2,4-triazolyl)]acetophenone (1.115g, 5mmol) and 10mL methanol into a 250mL round bottom flask, stir at room temperature , add KBH in batches under ice bath condition 4 (0.405g, 7.5mmol), the addition was completed, the ice bath was removed, stirred at 20-40°C, and the reaction progress was detected by TLC. After the reaction is complete, remove the solvent by rotary evaporation, add 15 mL of water, adjust the pH to 1-3 with concentrated hydrochloric acid, stir at room temperature for 1 h, and add 10% K 2 CO 3 Adjust the pH to 8-9, let stand, filter with suction, and wash with water (10 mL×3) to obtain IM1 as a white solid.
[0044] In a 100mL round bottom flask, add IM1 (0.672g, 3mmol), succinic anhydride (0.603g, 6mmol), acetone 6mL, Et 3 N 0.15mL, heated to reflux with stirring, and monitored the reaction progress by TLC. ...
Embodiment 2
[0050] Embodiment two, the synthesis of TM2 series compounds
[0051] The general route is as follows:
[0052]
[0053] Add NaH (0.813g, 20mmol) and THF 15mL to a 250mL three-necked flask, stir at room temperature; slowly add dropwise a solution of IM1 (2.245g, 10mmol) dissolved in THF 10mL with a constant pressure dropping funnel, and transfer to an 80°C oil bath for reaction 30 min; a solution of methyl 2-bromoacetate (1.735 g, 15 mmol) dissolved in THF 10 mL was slowly added dropwise. The progress of the reaction was monitored by TLC. Stirring was stopped, and the reaction mixture was concentrated under reduced pressure, poured into cold water (30 mL), and extracted with dichloromethane (2×20 mL). The organic extracts were combined, washed with saturated brine (2×20 mL), dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, purified by column chromatography (PE / EA=1 / 2), collected the eluent, evaporated to dryness under reduced pressure, V...
Embodiment 3
[0059] Embodiment three, the synthesis of TM3 series compound
[0060] The general route is as follows:
[0061]
[0062] In a 100mL round bottom flask, add Floxacin (2mmol), DCM 5mL and alkali (NaHCO 3 or Et 3 N, 3 mmol), stirred in an ice bath, 5 mL of a DCM solution of 1 mmol of solid phosgene (BTC) was added dropwise, stirred at room temperature, and the reaction progress was monitored by TLC. After the reaction, add 15 mL of saturated saline to adjust the pH to 4-5, separate the liquids, and wash with saturated saline (10 mL×3). The liquid was separated, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation to obtain intermediates IM3-1-IM3-8.
[0063] In a 100mL round bottom flask, add IM1 (1.2mmol), DCM 10mL, Et 3 N (1.5mmol) and DMAP (0.05mmol), stirred in an ice bath, added IM3-X (X = 1 ~ 8) (1mmol), warmed up to room temperature, continued to stir, TLC to monitor the reaction progress. After the reaction, add 15mL DCM, and was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com