Mononuclear copper complex and its preparation method and application
A copper complex and mononuclear technology, which is applied in the direction of copper organic compounds, drug combinations, antineoplastic drugs, etc., can solve the problems that the variety, quantity and function of natural nucleases cannot meet the needs of the market, and achieve good bonding and cleavage Effect, good anticancer activity, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Ligand L·2HClO 4 Synthesis:
[0031] Weigh 0.99g (5mmol) of dibenzylamine into a 50mL round bottom flask. Weigh 0.883g (5mmol) of 1-chloromethylnaphthalene, dissolve it in 20mL of acetone, and add it dropwise to dibenzylamine while stirring. Weigh 0.967g (7mmol) of K 2 CO 3 Particles were added to a round bottom flask to form a suspension. The reaction system was heated to reflux for 25h, and the material obtained by rotary evaporation was washed with H 2 O was dissolved and extracted with chloroform. The obtained extract was dried with MgSO4 and rotary evaporated to obtain 2.2723 g of the ligand product. Dissolve the oily liquid in 25mL of ethanol, then slowly add perchloric acid dropwise to produce a yellow-brown precipitate, filter it, and recrystallize it with absolute ethanol to obtain the ligand L 2HClO 4 The pale yellow powder 0.49g, yield 27.0%. Elemental analysis (%), theoretical value: (C 23 h 21 N 3 2HClO 4 ): C, 51.11; H, 3.89; N, 7.78. Experime...
Embodiment 2
[0033] Synthesis of mononuclear copper complex (1):
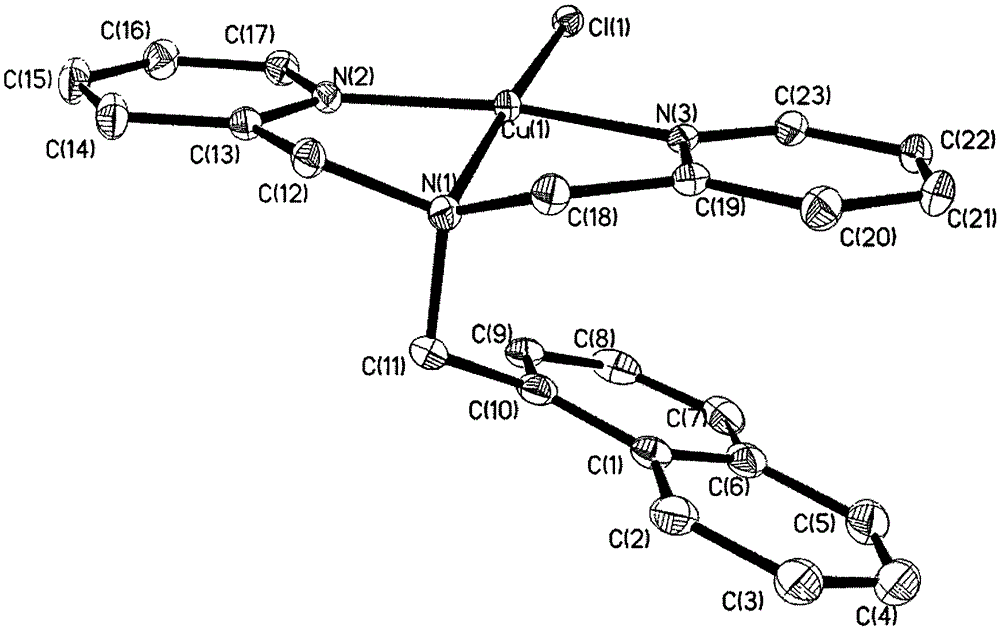
[0034] Weigh the ligand (2mmol) and dissolve it in 5mL ethanol, weigh copper trichloroacetate (2mmol), dissolve it with 5mL distilled water, add it to the reaction system, then add lithium hydroxide aqueous solution 5mL (5mmol), adjust the acidity of the system pH = 7. After stirring at room temperature for 4 hours, the reaction solution was filtered. After the filtrate was allowed to stand for 6 days, the precipitated crystals were the product, and the crystals were collected. Analyzed by X-ray single crystal diffractometer ( Figure 1a ) and elemental analysis, proved that the crystal is [CuLCl] (ClO4) (1). Determination of the percentage content of the corresponding elements (%): C, 51.43; H, 3.90; N, 7.86. The results are basically consistent with the theoretical values.
Embodiment 3
[0036] Synthesis of mononuclear copper complex (2):
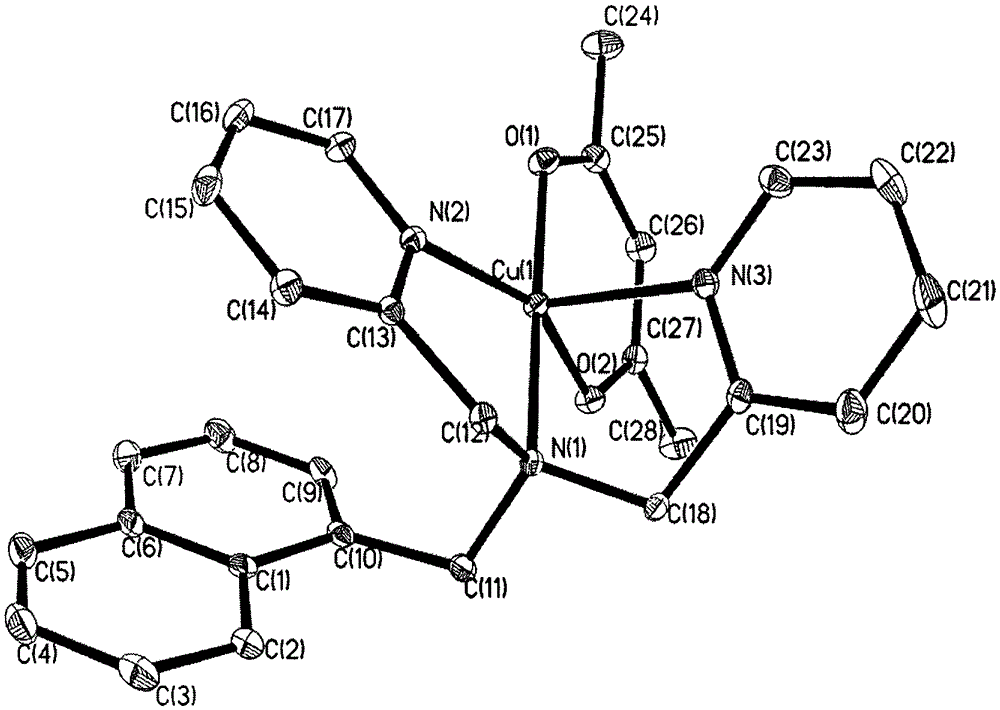
[0037] Take the ligand (2mmol) prepared in Example 1 and dissolve it in 5mL ethanol, weigh Cu(acac) 2 / CH 2 Cl 2 (2mmol), dissolved in 5mL of distilled water, added to the reaction system, and then 5mL (5mmol) of lithium hydroxide aqueous solution was added to adjust the acidity of the system to pH=7. After stirring at room temperature for 2.5h, add KPF 6 (4mmol), stirred for another 2h, and the reaction solution was filtered. After the filtrate was allowed to stand for 7 days, the precipitated crystals were the product, and the crystals were collected. Analyzed by X-ray single crystal diffractometer ( Figure 1b) and elemental analysis, it was proved that the crystal was [CuL(acac)](PF 6 )(2). Determination of the percentage content of the corresponding elements (%): C, 52.03; H, 4.37; N, 6.51. The results are basically consistent with the theoretical values.
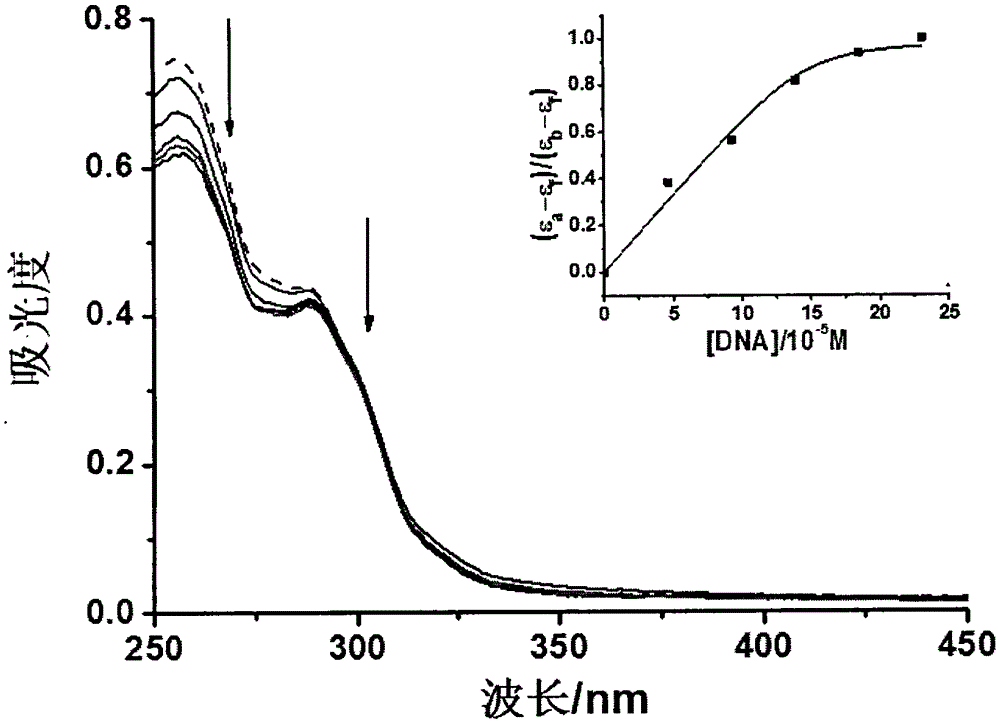
[0038] The structural parameters of the mononuclear c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com