Anti-VEGF monoclonal antibody and preparation method and application thereof
A monoclonal antibody and variable region technology, applied in the field of biomedicine, can solve the problems of high price, low affinity, and high price of bevacizumab
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Expression and Purification of Human VEGF Extracellular Region Recombinant Protein
[0059] Synthetic human VEGF-Fc fusion gene (synthesized by Nanjing GenScript Biotechnology Co., Ltd.), wherein the VEGF gene sequence is SEQ ID NO: 13, the Fc gene sequence is SEQ ID NO: 14, and the two sequences are connected to form VEGF-Fc For the fusion gene, design the forward and reverse primers as follows:
[0060] 5'-atccgccggcaagccgccaccatgaactttctgctgtcttgg-3' (SEQ ID NO: 15) and
[0061] 5'-tcactacgtatcatttacccggagacaggggagaggctc-3' (SEQ ID NO: 16), used to amplify the gene encoding VEGF-Fc fusion protein, introducing restriction endonuclease sites Ngo MIV and SnaBI at the upstream and downstream of the gene, PCR conditions 95°C for 10s, 60°C for 10s, 72°C for 15s, 35 cycles, and 72°C for 10min. The DNA polymerase was the product of TAKARA.
[0062] The PCR product was recovered by agarose gel (the recovery kit is a product of Omega bio-tek company), purified, and...
Embodiment 2
[0064] Example 2 Construction, expression, purification and identification of anti-VEGF human antibody eukaryotic expression vector
[0065] Antibody heavy chain (SEQ ID NO:1) and light chain (SEQ ID NO:7) genes were synthesized by Nanjing GenScript Biotechnology Co., Ltd. Use PCR reaction to amplify the coding DNA sequences of the heavy chain and light chain above, and introduce appropriate enzyme cutting sites at both ends of the gene of the heavy chain and light chain of the antibody. Ngo MIV and Sna BI restriction sites were introduced respectively, and the sequences of the forward and reverse primers were:
[0066] 5'-ACCTGCCGGCAAGCCGCCACCATGGGTTGGAGCCTCATCTTGCTCTTC-3' (SEQ ID NO: 17) and
[0067] 5'-GCTCTACGTATCATTTACCTGGAGACAGGGAGAGGC-3' (SEQ ID NO: 18), Ngo MIV and SnaB I were respectively introduced at the 5' end and 3' end of the antibody light chain variable region gene, and the forward and reverse primer sequences were respectively:
[0068] 5'-ACCTGCCGGCAAGCCGCC...
Embodiment 3
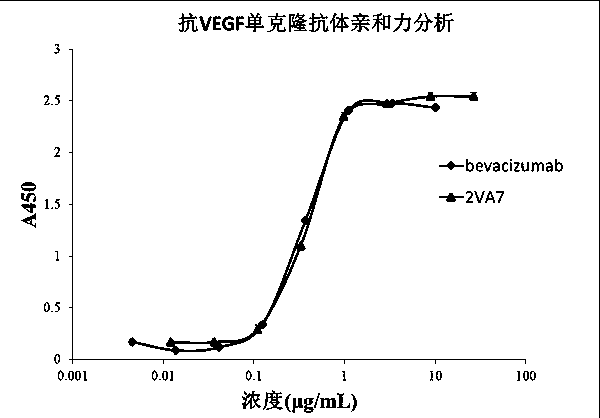
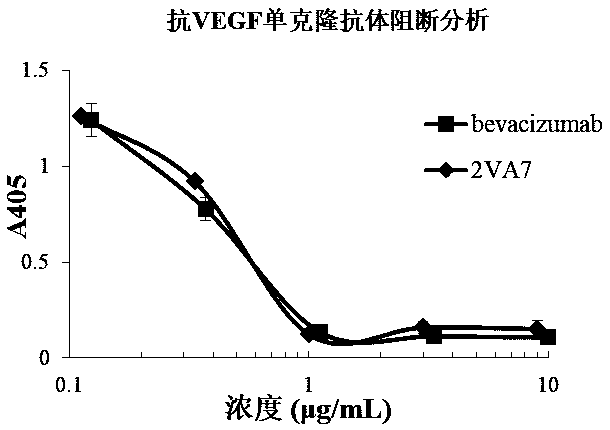
[0073] Example 3 Affinity Identification of Anti-VEGF Human Antibody
[0074]Antibody affinity identification. The VEGF recombinant protein prepared in Example 1 was coated onto an ELISA plate at 2 µg / ml (working volume 30 µL), and left standing at 4°C overnight. Wash 3 times with regular phosphate buffered saline (PBST) containing 0.05% Tween 20. Block with 5% skimmed milk for 1 hour at room temperature. Wash 3 times with PBST, prepare the monoclonal antibody 2VA7 prepared in Example 2 to a concentration of 26.5 µg / ml, and perform 3-fold serial dilutions, a total of 8 gradients, add 30 µl to each well, and let stand at room temperature for 1 hour. Wash 3 times with PBST, add 30 μl 1:4000 dilution of horseradish peroxidase-labeled goat anti-human light chain constant region secondary antibody (Millipore company product), and let stand at room temperature for 1 hour. Wash 4 times with PBST, add TMB for color development, and use 2M H 2 SO 4 Stop the reaction. Read at 450n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com