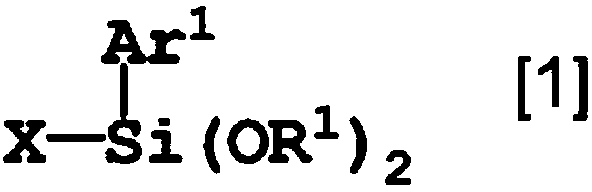Low-viscosity agent for polymerizable compound having high refractive index and polymerizable composition containing same
A technology of polymeric compounds and polymeric compositions, which can be used in instruments, optics, optical components, etc., and can solve problems such as deterioration of operability, increased viscosity of materials, and high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0218] Hereinafter, although an Example is given and this invention is demonstrated more concretely, this invention is not limited to a following Example.
[0219] In addition, in Examples, the apparatus and conditions used for preparation of a sample and analysis of a physical property are as follows.
[0220] (1) Spin coater
[0221] Device: Cee (registered trademark) 200X manufactured by Brewer Science
[0222] (2) UV exposure
[0223] Device: Intermittent UV irradiation device manufactured by Aigraphy Co., Ltd. (high pressure mercury lamp 2kW x 1 lamp)
[0224] (3) 1 H NMR spectrum
[0225] Installation: AVANCE III HD by Bruker
[0226] Measurement frequency: 500MHz
[0227] Solvent: CDCl 3
[0228] Internal reference: Tetramethylsilane (δ = 0.00ppm)
[0229] (4) Gel Permeation Chromatography (GPC)
[0230] Device: Prominence (registered trademark) GPC system manufactured by Shimadzu Corporation
[0231] Column: Shodex (registered trademark) GPC KF-804L and GPC ...
manufacture example 1
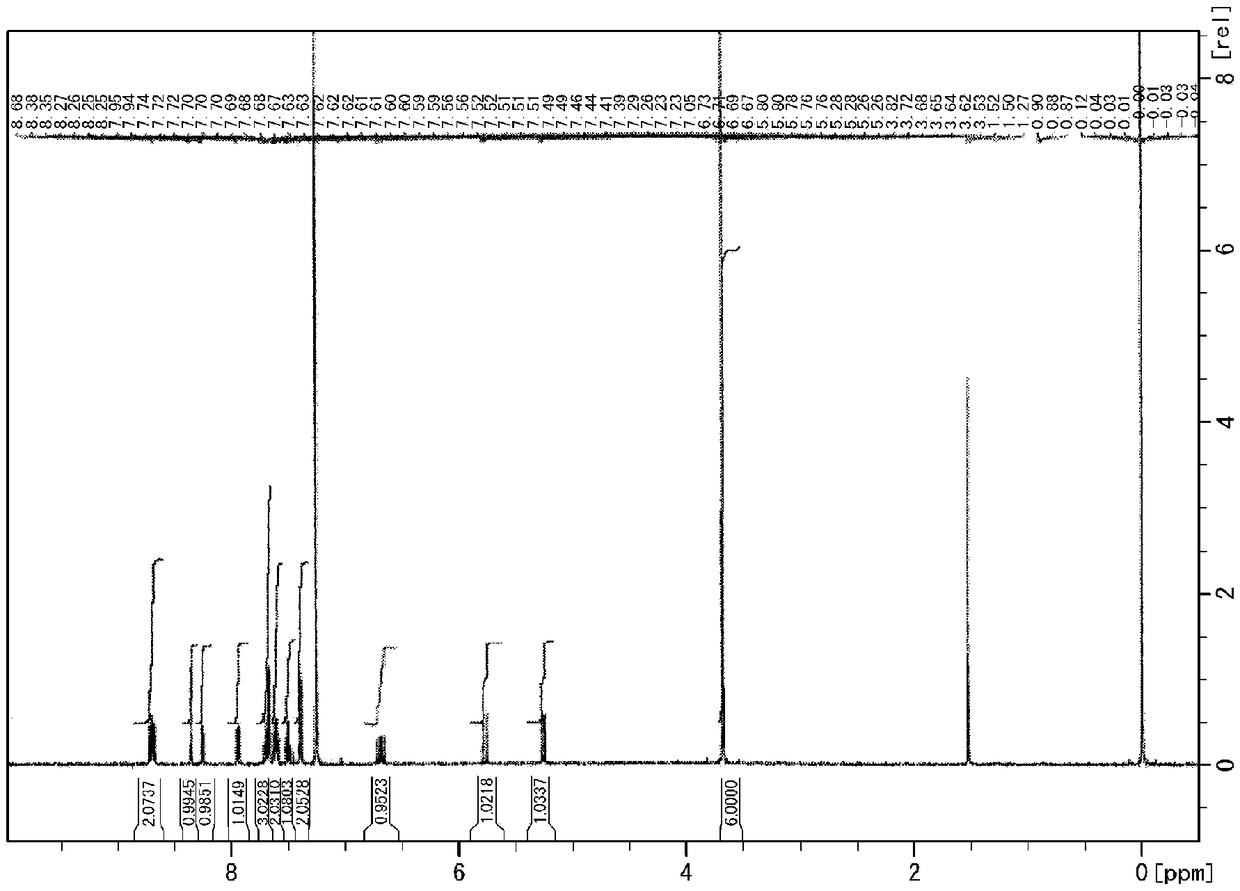
[0267] [Production Example 1] Production of Dimethoxy(phenanthrene-9-yl)(4-vinylphenyl)silane (SPeDMS)
[0268] Into a 1 L reaction flask equipped with a condenser, 15.7 g (0.65 mol) of magnesium chips [manufactured by Kanto Chemical Co., Ltd.] was placed, and the air in the flask was replaced with nitrogen gas using a nitrogen balloon. A mixture of 151.2 g (0.58 mol) of 9-bromophenanthrene [manufactured by Tokyo Chemical Industry Co., Ltd.] and 518 g of THF was added dropwise thereto at room temperature (about 23° C.) over 1 hour, and stirred for further 1 hour to prepare Grignard reagent.
[0269] Into a 2L reaction flask, 131.9 g (0.58 mol) of STMS and 259 g of THF were charged, and the air in the flask was replaced with nitrogen gas using a nitrogen balloon. The above-mentioned Grignard reagent was added dropwise thereto over 30 minutes under reflux (about 66° C.), and further refluxed for 24 hours. THF was distilled off from the reaction mixture under reduced pressure u...
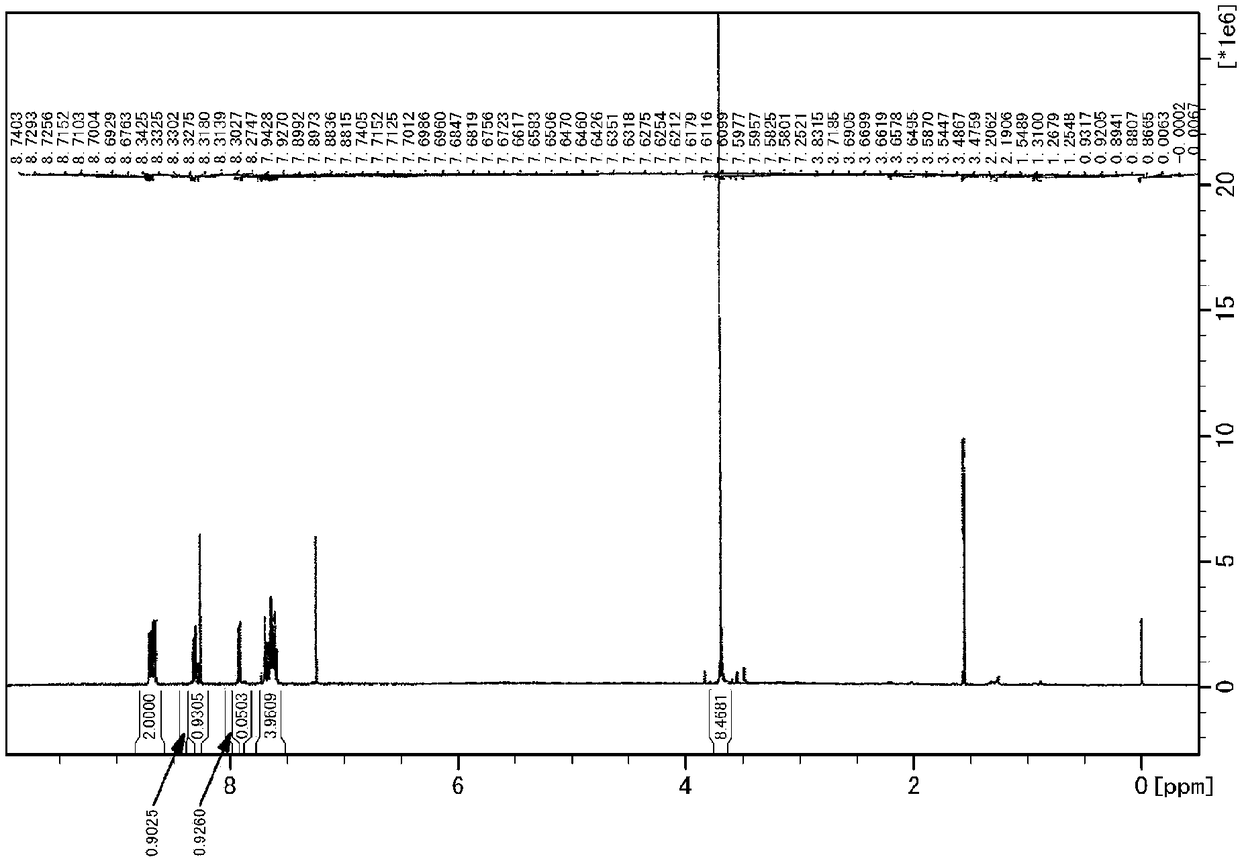
manufacture example 2
[0271] [Production Example 2] Production of Naphthalene-2-ylmethyl Acrylate (NMA)
[0272] Into a 200 mL reaction flask, 25.0 g (0.158 mol) of 2-naphthylmethanol [manufactured by Tokyo Chemical Industry Co., Ltd.] and 158 g of THF were added, and the air in the flask was replaced with nitrogen using a nitrogen balloon, followed by cooling to 0°C. 17.58 g (0.174 mol) of triethylamine [manufactured by Tokyo Chemical Industry Co., Ltd.] and 15.73 g (0.174 mol) of acryloyl chloride [manufactured by Tokyo Chemical Industry Co., Ltd.] were added thereto, and stirred at room temperature (about 23° C.) 1 hour. 158 g of water was added to the reaction mixture, and the product was extracted with 158 g of ethyl acetate. The solvent was distilled off from the organic layer under reduced pressure using an evaporator to obtain a crude product. The crude product was purified by silica gel chromatography (hexane / ethyl acetate=9 / 1 (mass ratio)) to obtain 21.5 g of naphthalene-2-ylmethyl acryla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com