Cholesteric-phase liquid crystal material with color controlled by visible light
A cholesteric liquid crystal and visible light technology, applied in liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of unfavorable on-demand design, limited application, high energy of ultraviolet light, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the cholesteric liquid crystal material of the present invention is as follows: 0.9 to 50 parts by weight of the unsubstituted azobenzene derivative, 0.1 to 50 parts by weight of the substituted chiral azobenzene derivative After being mixed with 30-99 parts of the nematic liquid crystal, it is packed into a liquid crystal cell with an alignment structure inside to form a cholesteric liquid crystal material.
[0043] The present invention also provides a preparation method of substituted chiral azobenzene derivatives. The preparation of the substituted chiral azobenzene derivatives can realize the selectivity of intermediate products by changing reaction conditions such as reaction time and solvent type Reaction, and then the substituted chiral azobenzene derivatives of different structures can be obtained under control, comprising the following steps:
[0044] (1) will be dissolved in the solvent A Mixed with the aqueous solution B of inorga...
Embodiment 1
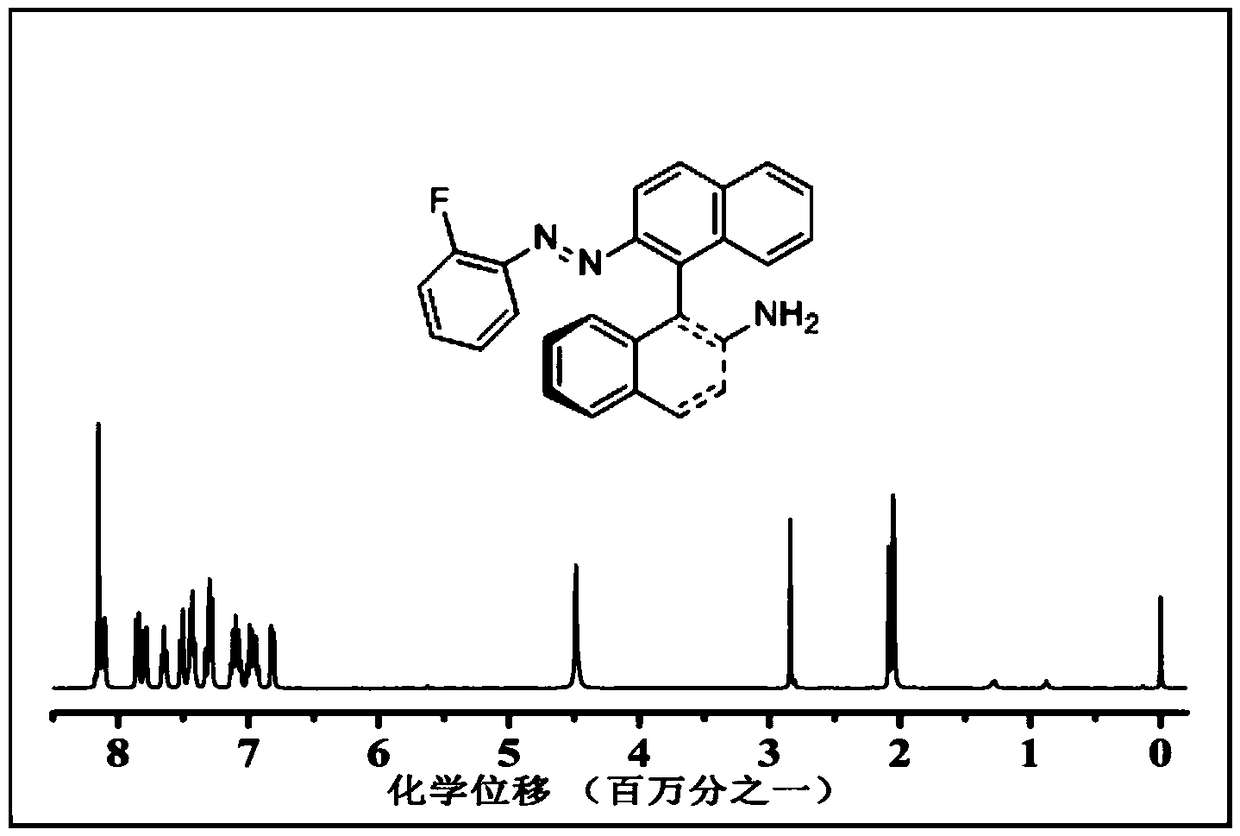
[0055] (1) Dissolve 0.28g of 2-fluoroaniline in 10ml of tetrahydrofuran to obtain A, add A to 3.22g of sodium nitrite aqueous solution B at a rate of 1 μl, react under air atmosphere at 20°C, and react with magnetic stirring for 4.0 h, the product was extracted with water and dichloromethane, and the dichloromethane phase was spin-dried to obtain reaction intermediate 1;
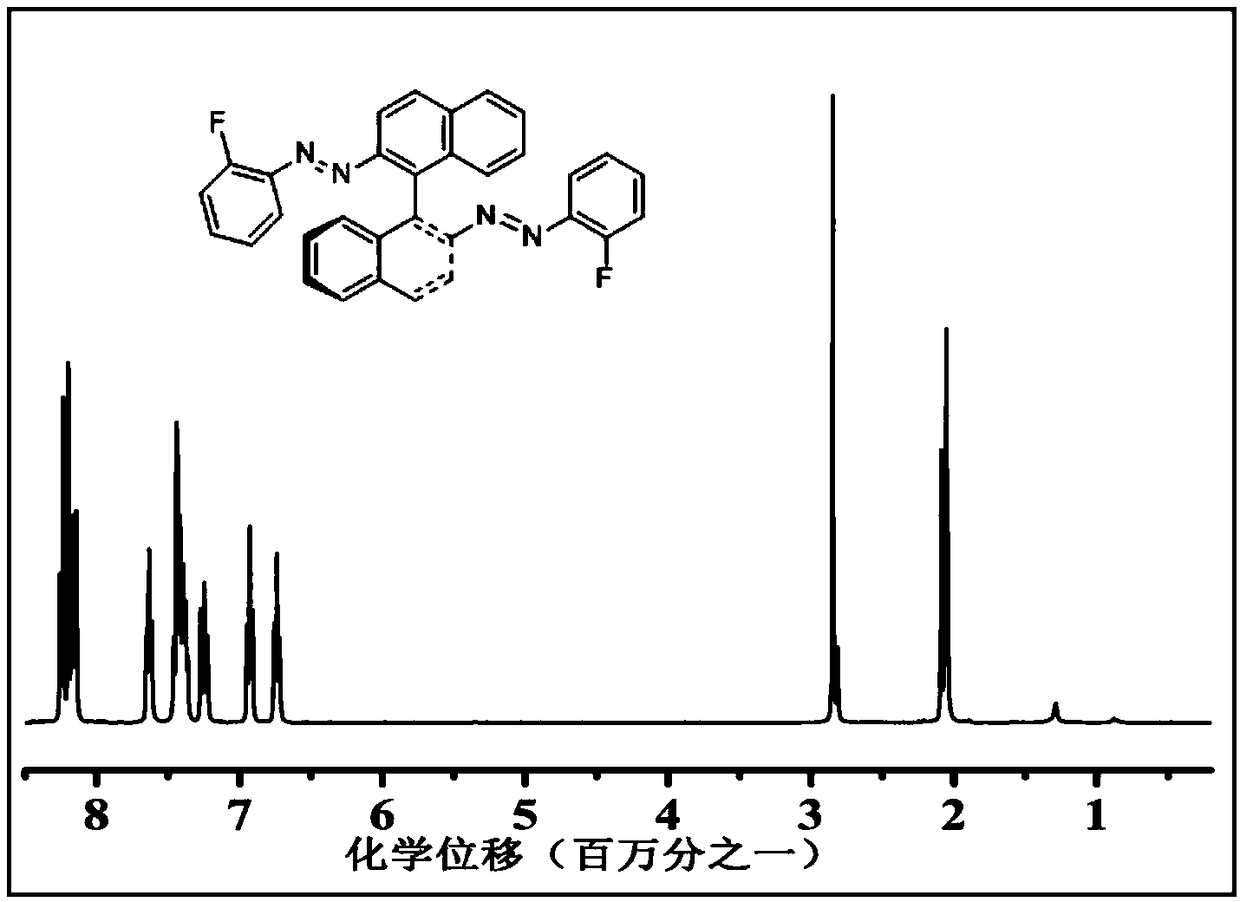
[0056] (2) Dissolve 0.26g of reaction intermediate 1 in a mixed solvent of 10ml of acetonitrile and petroleum ether (volume ratio 1:1), then add 0.35g of (S)-(-)-1,1 ’ In -bin-dinaphthylamine, under an argon atmosphere at 25°C, the magneton was stirred for 0.5h, and after filtration and column chromatography, a substituted chiral azobenzene derivative was obtained, and its structural formula was:
[0057]
[0058] (3) 0.3g of the above substituted chiral azobenzene derivatives, 0.36g of unsubstituted azobenzene derivatives and 0.3g nematic liquid crystal (5CB) After mixing, pack into a liquid crystal ...
Embodiment 2
[0063] (1) Dissolve 0.51g of 2,6-dibromoaniline in 21ml of chloroform to obtain A, add A to 6.52g of potassium iodide aqueous solution B at a rate of 40μl, react under an argon atmosphere at 60°C, and mechanically Stirring and reacting for 26h, the product obtained a reaction intermediate after filtration and column chromatography;
[0064] (2) Dissolve 0.25g of the reaction intermediate in 10ml of carbon tetrachloride, then add 0.58g of (R)-(-)-1,1'-bin-dinaphthylamine, at 60°C under a nitrogen atmosphere , mechanically stirred for 120.0h, after filtration and column layer, a substituted chiral azobenzene derivative was obtained, and its structural formula was:
[0065]
[0066] (3) 0.002g substituted chiral azobenzene derivatives, 0.018g unsubstituted azobenzene derivatives and 1.98g nematic liquid crystal After mixing, it is packed into a liquid crystal cell with an alignment structure inside to form a cholesteric liquid crystal material;
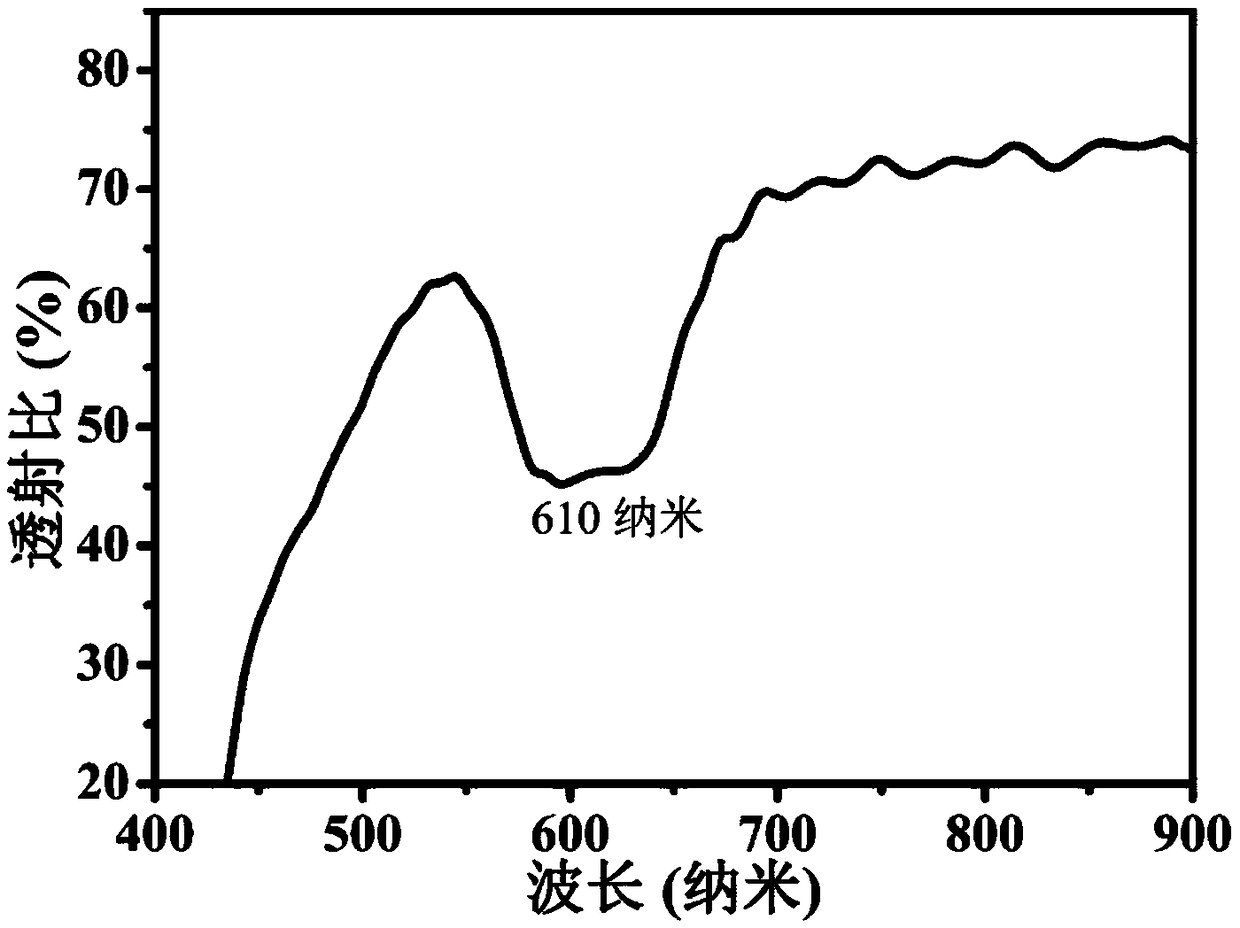
[0067] (4) Use a light in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com