A kind of camptothecin prodrug based on cationic amino acid modification and its preparation method, nano drug-loaded particle and its application
A cationic amino acid and camptothecin technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of affecting drug efficacy, difficult loading, poor water solubility, etc. Achieve excellent stability and good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention provides a kind of preparation method of the camptothecin prodrug based on cationic amino acid modification described in above-mentioned technical scheme, comprises the following steps:
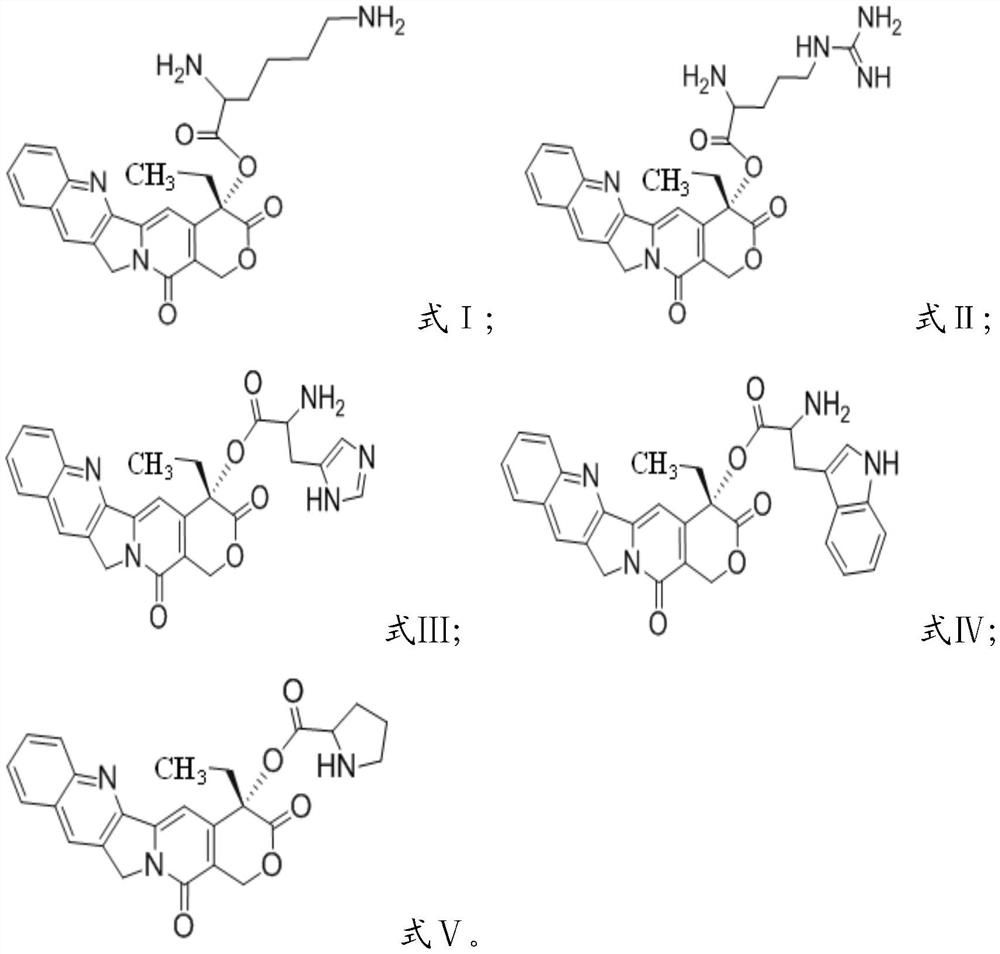
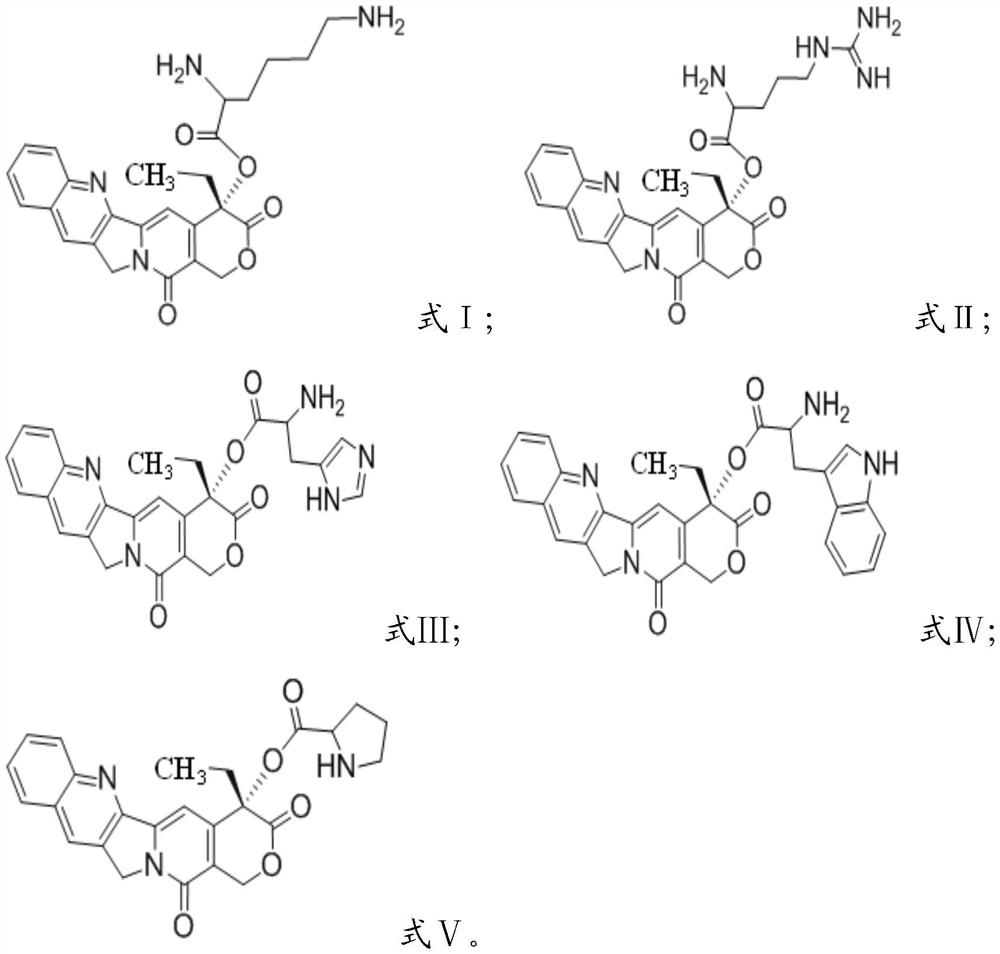
[0024] Condensation reaction of camptothecin and group-protected amino acid in the presence of condensing agent and solvent, deprotection, to obtain camptothecin prodrug based on cationic amino acid modification, having formula I, formula II, formula III, formula IV or Formula V structure;
[0025] The amino acid protected by the group is selected from bis-tert-butoxycarbonyl protected lysine, tert-butoxycarbonyl and 2,2,4,6,7-pentamethyl-dihydrobenzofuran-5-sulfonyl Protected arginine, bis-tert-butoxycarbonyl-protected histidine, bis-tert-butoxycarbonyl-protected tryptophan or tert-butoxycarbonyl-protected proline.
[0026] The present invention preferably carries out the condensation reaction under anhydrous conditions. In the present invention, the temperatur...
Embodiment 1
[0045] Preparation of Cationic Amino Acid Modified Camptothecin Prodrug
[0046] (1) Under anhydrous conditions, combine camptothecin (CPT) with bis-tert-butoxycarbonyl-protected lysine (Boc-Lys(Boc)-OH), tert-butoxycarbonyl, 2,2,4 , 6,7-pentamethyl-dihydrobenzofuran-5-sulfonyl protected arginine (Boc-Arg(Pbf)-OH), bis-tert-butoxycarbonyl protected histidine (Boc-His (Boc)-OH), bis-tert-butoxycarbonyl-protected tryptophan (Boc-Trp(Boc)-OH) and tert-butoxycarbonyl-protected proline Pro(Boc)-OH)) according to the molar ratio of 1 : 1.2 Disperse in a certain volume of dichloromethane, and add 4-dimethylaminopyridine (DMAP) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt of 2 times the amount of amino acid Condensation reaction of hydrochloric acid (EDC HCl) at room temperature, the reaction time is 2 days, then remove dichloromethane, add trifluoroacetic acid, deprotect, and settle in 10 times the volume of ether, the obtained solid is amino acid Modified camptothecin pr...
Embodiment 2
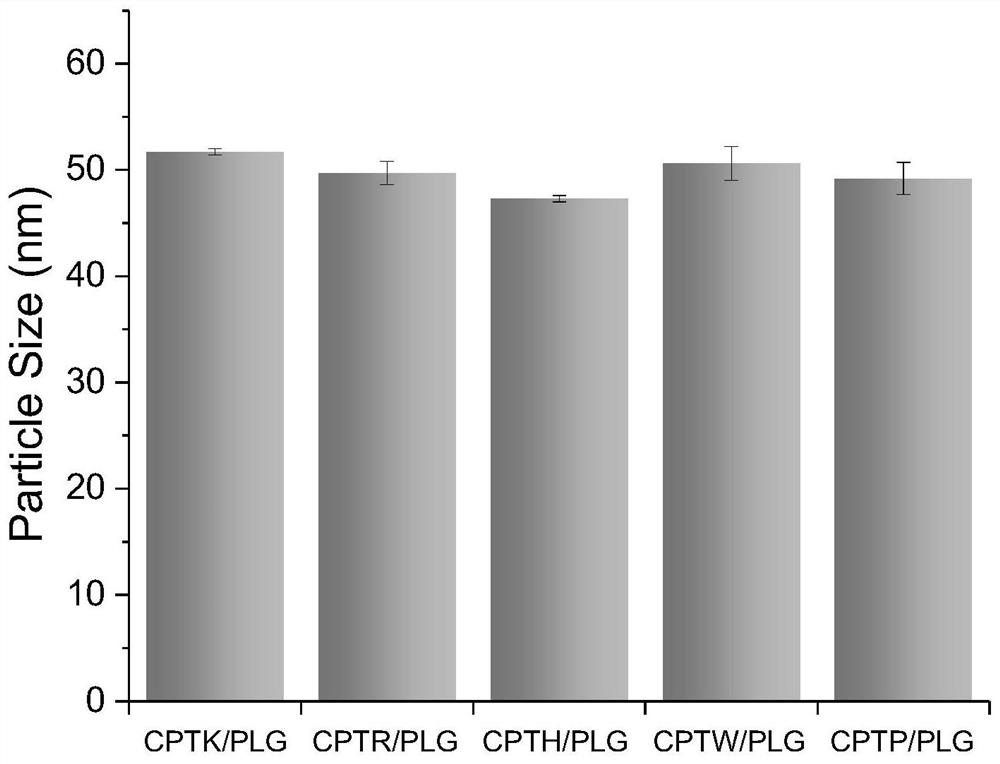
[0054] Embodiment 2: the drug loading capacity of the modified camptothecin of cationic amino acid
[0055] CPTK, CPTR, CPTH, CPTW, and CPTP provided by the present invention can be electrostatically compounded with PLG to obtain higher drug loading efficiency (drug loading %=loaded CPT mass / (loaded CPT mass+carrier mass)*100 %). CPTK / PLG, CPTR / PLG, CPTH / PLG, CPTW / PLG, and CPTP / PLG. Its preparation and detection methods are as follows:
[0056] Prepare a certain concentration of CPTK, CPTR, CPTH, CPTW, CPTP aqueous solution (solution X), the concentration of which is 5 mg / mL. and an aqueous solution of PLG at a concentration of 5 mg / mL (solution Y). Take certain mentioned X solution and Y solution and mix them according to different mass ratios, such as X / Y=1 / 1, 1 / 2, 1 / 4, and vortex evenly to form nanoparticles. Each complex solution was dialyzed against acidic water (pH=5) with a 3500Da dialysis bag for 24 hours, the solution in the dialysis bag was taken out, after freez...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com