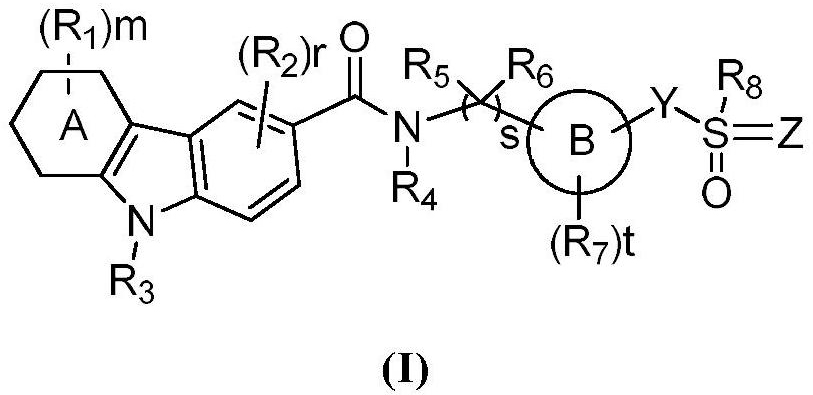
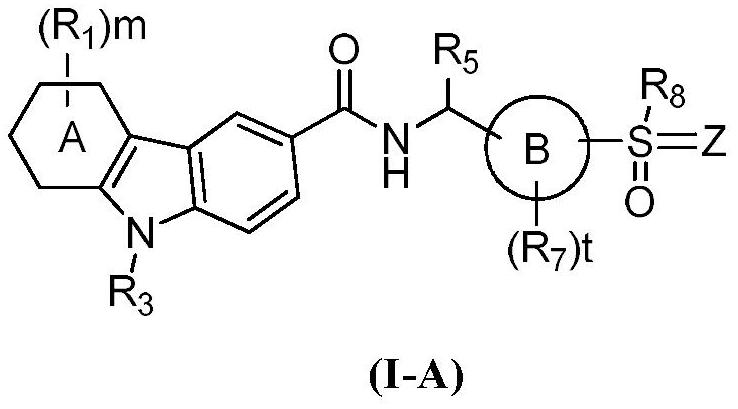
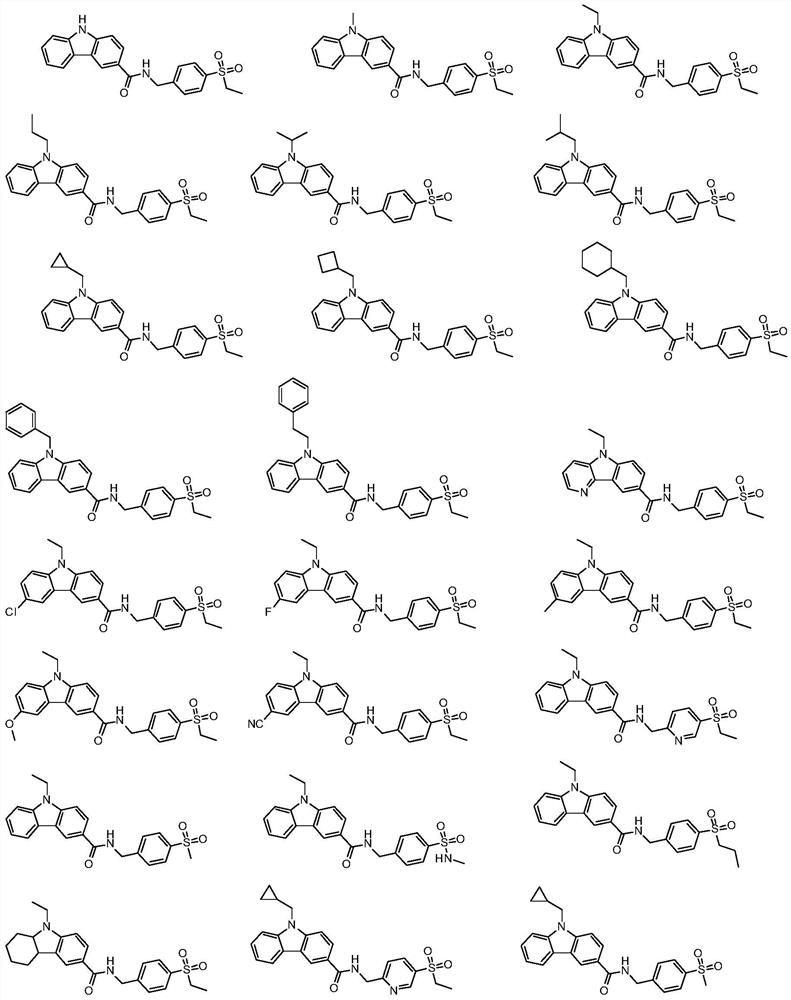
Carbazole amide derivatives or salts thereof, preparation method and use thereof
A technology of carbazole amide and derivatives, applied in the field of chemical medicine, can solve the problem of ignorance of Th17 function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: 9-Ethyl-N-(4-(ethylsulfonyl)phenyl)-9H-carbazole-3-carboxamide
[0086] 9-ethyl-N-(4-(ethylsulfonyl)benzyl)-9H-carbazole-3-carboxamide
[0087]
[0088] Intermediate 1: Synthesis of 9-ethyl-9H-carbazole-3-carboxylic acid
[0089] Step 1: Synthesis of methyl 4-anilinobenzoate
[0090] To a 25mL microwave tube was added aniline (1.82g, 19.5mmol), methyl 4-bromobenzoate (3.5g, 16.28mmol), potassium carbonate (6.72g, 49.4mmol), rac-BINAP (506mg, 0.81mmol), Palladium acetate (218mg, 0.97mmol), toluene (10mL), microwave heating reaction at 130°C for 2 hours, after the reaction is complete, dilute with ethyl acetate, filter with diatomaceous earth, spin dry the solvent under reduced pressure, and separate on a silica gel column (petroleum ether: acetic acid Ethyl ester=10:1-5:1) to obtain 3.5 g of a yellow solid product with a yield of 94.6%. 1 H NMR (400MHz, CDCl 3 )δ7.92(d, J=8.5Hz, 2H), 7.34(t, J=7.7Hz, 2H), 7.17(d, J=7.7Hz, 2H), 7.07(t, J=7.3Hz, 1H) , 6...
Embodiment 2
[0104] Example 2: 9-Ethyl-N-(4-ethylsulfonyl)benzyl)-2,3,4,9-tetrahydro-1H-carbazole-6-carboxamide (9-ethyl-N-(4-(ethylsulfonyl)benzyl)-2,3,4,9-tetrahydro-1H-carbazole-6-carboxamide)
[0105]
[0106] Step 1: Synthesis of 2,3,4,9-tetrahydro-1H-carbazole-6-carboxylic acid
[0107] Add 4-hydrazinobenzoic acid (1g, 6.6mmol), cyclohexanone (647mg, 6.6mmol), 1,4-dioxane (10mL), concentrated hydrochloric acid (5mL) into a 25mL single-necked bottle, and reflux at 120°C After reacting overnight, a large amount of solids were produced. After filtration, the obtained solids were put into water (10 mL) and stirred for 30 minutes, and then filtered. The obtained solids were vacuum-dried to obtain 1.13 g of a brown solid product, with a yield of 80.1%. MS(ESI)m / z:216.1(MH + ).
[0108] Step 2: Synthesis of N-(4-(ethylsulfonyl)benzyl)-2,3,4,9-tetrahydro-1H-carbazole-6-carboxamide
[0109] Add 2,3,4,9-tetrahydro-1H-carbazole-6-carboxylic acid (169mg, 0.78mmol), 4-(ethylsulfonyl)benzy...
Embodiment 3
[0112] Example 3: N-(4-(ethylsulfone)benzyl)-9H-carbazole-3-carboxamide
[0113] N-(4-(ethylsulfonyl)benzyl)-9H-carbazole-3-carboxamide
[0114]
[0115] Step 1: Synthesis of methyl 4-(phenylamino)benzoate
[0116] Under the protection of argon, add aniline (1.82g, 19.53mmol), methyl 4-bromobenzoate (3.5g, 16.28mmol), palladium acetate (218mg, 0.97mmol), rac-BINAP (506mg , 0.81mmol), potassium carbonate (6.72g, 48.62mmol), toluene (10mL). Microwave at 160°C for 2 hours and cool to room temperature. After the reaction, the solvent was spin-dried under reduced pressure, dichloromethane was added to dilute and filtered. After the organic phase was spin-dried, water (15 mL) was added, extracted with ethyl acetate (3×30 mL), washed with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and spin-dried under reduced pressure to obtain a crude product. Separated by column chromatography, 3.32 g of a light brown solid was obtained, with a yield of 89.7%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com