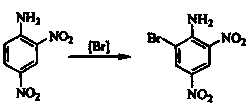
A new bromination process of 2,4-dinitroaniline
A technology of dinitroaniline and new process, applied in the field of pharmaceutical intermediates, can solve the problems of high bromine corrosiveness and toxicity, cannot meet industrial needs, and the reaction operation is cumbersome, and achieves simple operation, easy separation, and promotes the reaction to proceed. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 36.8g of 1,4-butane sultone, add 300ml of ethyl acetate into the reactor, install a constant pressure dropping funnel, a magnetic stirrer and a reflux condenser, and heat up to 60°C in a water bath. Slowly add 24.6g of N-methylimidazole at ℃, after the dropwise addition is completed, keep the system warm at 80℃ for 2 hours until a white precipitate is produced; filter the system under reduced pressure, wash the filter cake with ethyl acetate, and put it in an oven Drying at 100°C, the resulting product is 1-methyl-3-(4-sulfonic acid) butyl imidazolium salt; add water to the 1-methyl-3-(4-sulfonic acid) butyl imidazolium salt Dissolve, add hexafluorophosphoric acid to react at 80°C, and then remove the water to obtain a light yellow viscous liquid product, which is 1-methyl-3-(4-sulfonic acid) butylimidazole hexafluorophosphate ionic liquid catalyst .
Embodiment 2
[0028] In a four-neck flask equipped with a stirrer, a thermometer and a constant pressure dropping funnel, add 150 g of tetrahydrofuran, 150 g of 30% hydrogen peroxide, 13 g of 1-methyl-3-(4-sulfonic acid) butylimidazole six Fluorophosphate, start the stirring device, drop 180g of 60% sodium bromide solution and 30.5g of 2,4-dinitroaniline at the same time under the condition of 50°C, control the dropping speed so that the two drop simultaneously within 5 hours After the heat preservation reaction was completed for about 4 hours, the temperature was raised to 60 ° C, and the temperature was maintained for 5 hours, then the temperature was raised to 90 ° C, and the temperature was maintained for 3 hours to obtain a 6-bromo-2,4-dinitroaniline solution. Separate the 1-methyl-3-(4-sulfonic acid group) butylimidazolium hexafluorophosphate ionic liquid layer and the product layer, wash the product layer with water until neutral, and drain to obtain 6-bromo-2,4-di The crude product ...
Embodiment 3
[0030] In a four-necked flask equipped with a stirrer, a thermometer and a constant pressure dropping funnel, add 1 kg of ethyl acetate, 1 kg of 30% hydrogen peroxide, 0.2 kg of 1-methyl-3-(4-sulfonic acid) butyl imidazole hexafluorophosphate, start the stirring device, and drop 1.8kg of 50% sodium bromide solution and 0.3kg of 2,4-dinitroaniline at the same time under the condition of 50°C, and control the rate of addition so that the two are within 5 The drops were completed within 1 hour, and the temperature was raised to 60°C after the heat preservation reaction for about 4 hours, and the heat preservation was 5 hours. Set the layers, separate the 1-methyl-3-(4-sulfonic acid group) butylimidazolium hexafluorophosphate ionic liquid layer and the product layer, wash the product layer with water to neutrality, and drain to obtain 6-bromo-2 , 4-dinitroaniline crude product, add 3 times the weight of the crude product, soak in ethanol with a volume percentage concentration of 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com