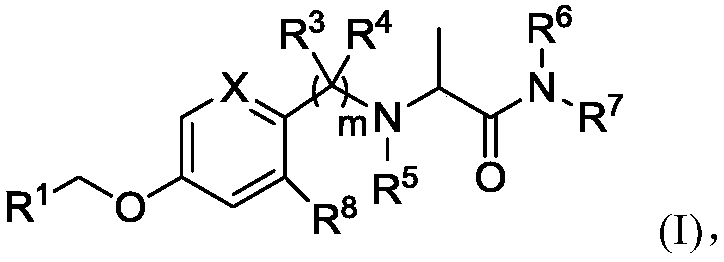
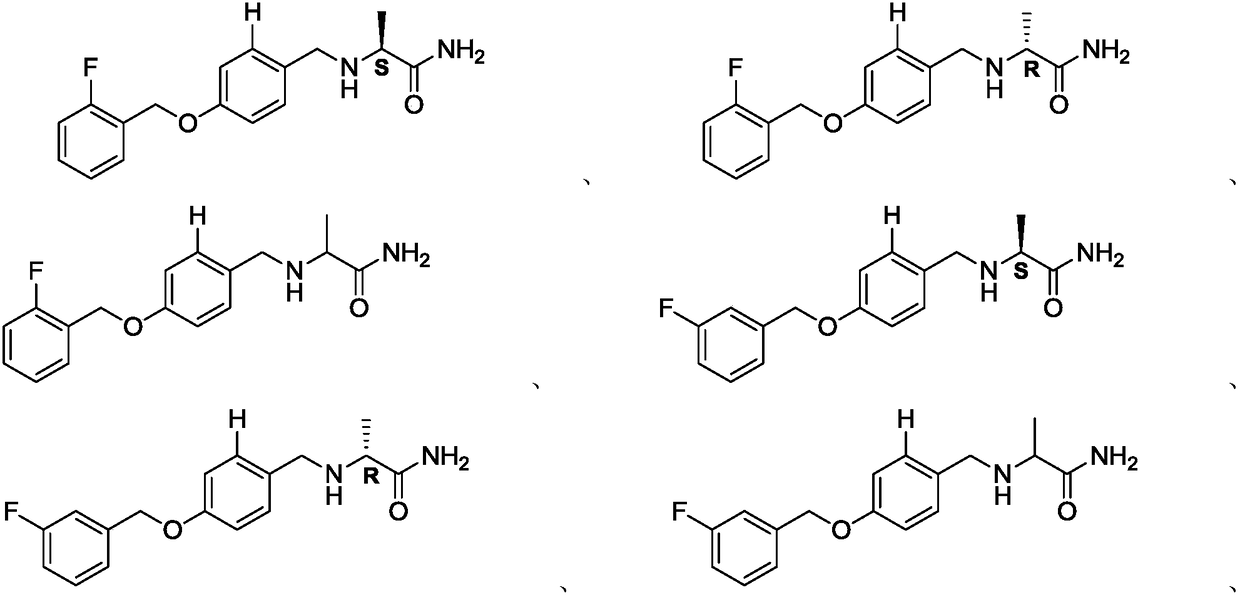
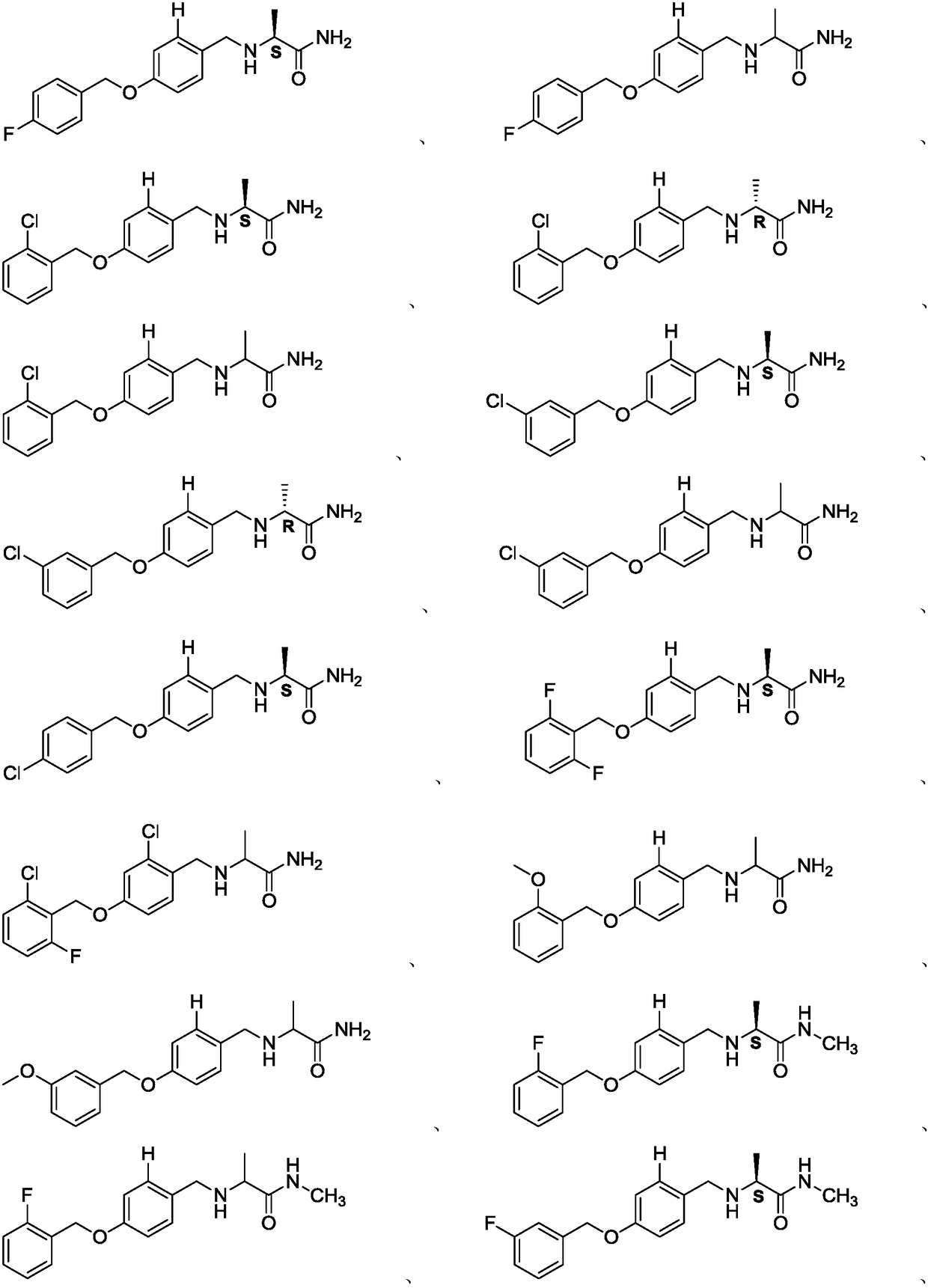
Alpha-aminoamide derivative and application thereof
An alkyl and hydroxyl technology, applied in the direction of drug combinations, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve problems that cannot delay disease progression, dyskinesias, motor fluctuations, and mental symptoms, and achieve good results. Effect of pharmacodynamic and pharmacokinetic properties, good safety profile, good metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Embodiment 1 (S)-2-((4-(4-(methylsulfonyl) methyl) benzyloxy group) benzyl amino) the synthesis of propanamide
[0245]
[0246] Step 1) Synthesis of 4-(4-(bromomethyl)benzyloxy)benzaldehyde
[0247] 4-Hydroxybenzaldehyde (1.0g, 8.19mmol), 1,4-bis(bromomethyl)benzene (4.3g, 16.38mmol), potassium carbonate (4.5g, 32.76mmol) and acetone (50mL) were added to In a 100mL single-necked round bottom flask, react at 60°C for 5h, cool to room temperature, filter, collect the filtrate, and then directly mix the sample for column chromatography purification (petroleum ether / ethyl acetate (v / v)=5 / 1) to obtain the title compound As a white solid (1.35 g, 54.0%).
[0248] MS(ESI,pos.ion)m / z:305.0[M+H] + ;
[0249] 1 H NMR (400MHz, CDCl 3 )δ(ppm)9.89(s,1H),7.92-7.75(m,2H),7.46-7.39(m,4H),7.07(d,J=8.7Hz,2H),5.15-5.14(m,2H) ,4.50(s,2H).
[0250] Step 2) Synthesis of 4-(4-((methylsulfonyl)methyl)benzyloxy)benzaldehyde
[0251] 4-(4-(Bromomethyl)benzyloxy)benzaldehyde (0.78...
Embodiment 2
[0259] Synthesis of Example 2 (S)-2-(4-(4-((methylsulfonyl)methyl)benzyloxy)benzyl)(propyl-2-yn-1-yl)amino)propionamide
[0260]
[0261] (S)-2-((4-(4-(Methylsulfonyl)methyl)benzyloxy)benzylamino)propanamide (0.16g, 0.43mmol), DIPEA (0.34mL, 2.04mmol), DMF (5mL) and 3-bromopropyne (0.29mL, 3.44mmol) were successively added into a 100mL single-necked round bottom flask, heated to 80°C for 15h, stopped the reaction, cooled to room temperature, added water (20mL) and washed with ethyl acetate (20mL ×2) extraction, washing with water (20mL×3), collecting the ethyl acetate layer, and then concentrating and purifying by column chromatography (petroleum ether / ethyl acetate (v / v)=1 / 5) to obtain the title compound as a white solid (0.05g ,28.4%).
[0262] MS(ESI,pos.ion)m / z:415.2[M+H] + ;
[0263] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.45 (dd, J = 19.4, 7.8Hz, 4H), 7.24 (d, J = 8.2Hz, 2H), 6.93 (d, J = 8.3Hz, 2H), 5.07 (s, 2H), 4.26(s,2H),3.63(dd,J=75.3,13.1Hz,2H),3.42(dd,J=13.7,6...
Embodiment 3
[0265] Embodiment 3 (S)-2-(4-(2,2,3,3-tetrafluoropropoxy) benzylamino) propionamide synthesis
[0266]
[0267] Step 1) Synthesis of 4-(2,2,3,3-tetrafluoropropoxy)benzaldehyde
[0268] Tetrafluoropropyl 4-methylbenzenesulfonate (4.0g, 13.97mmol), 4-hydroxybenzaldehyde (3.4g, 27.95mmol), potassium carbonate (3.86g, 27.95mmol) and DMF (20mL) were added to In a 100mL single-necked round-bottom flask, heat to 100°C for 13h, stop the reaction, cool to room temperature, add water (40mL) and extract with ethyl acetate (50mL×2), wash with water (50mL×3), collect the ethyl acetate layer, and then Purification by concentration column chromatography (petroleum ether / ethyl acetate (v / v)=10 / 1) gave the title compound as a light yellow oil (3.10 g, 94.0%).
[0269] MS(ESI,pos.ion)m / z:237.1[M+H] + ;
[0270] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 9.92 (s, 1H), 7.88 (d, J = 8.8Hz, 2H), 7.05 (d, J = 8.7Hz, 2H), 6.06 (tt, J = 53.1, 4.6Hz, 1H), 4.44(t,J=11.8Hz,2H).
[0271] Step 2) Synth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com