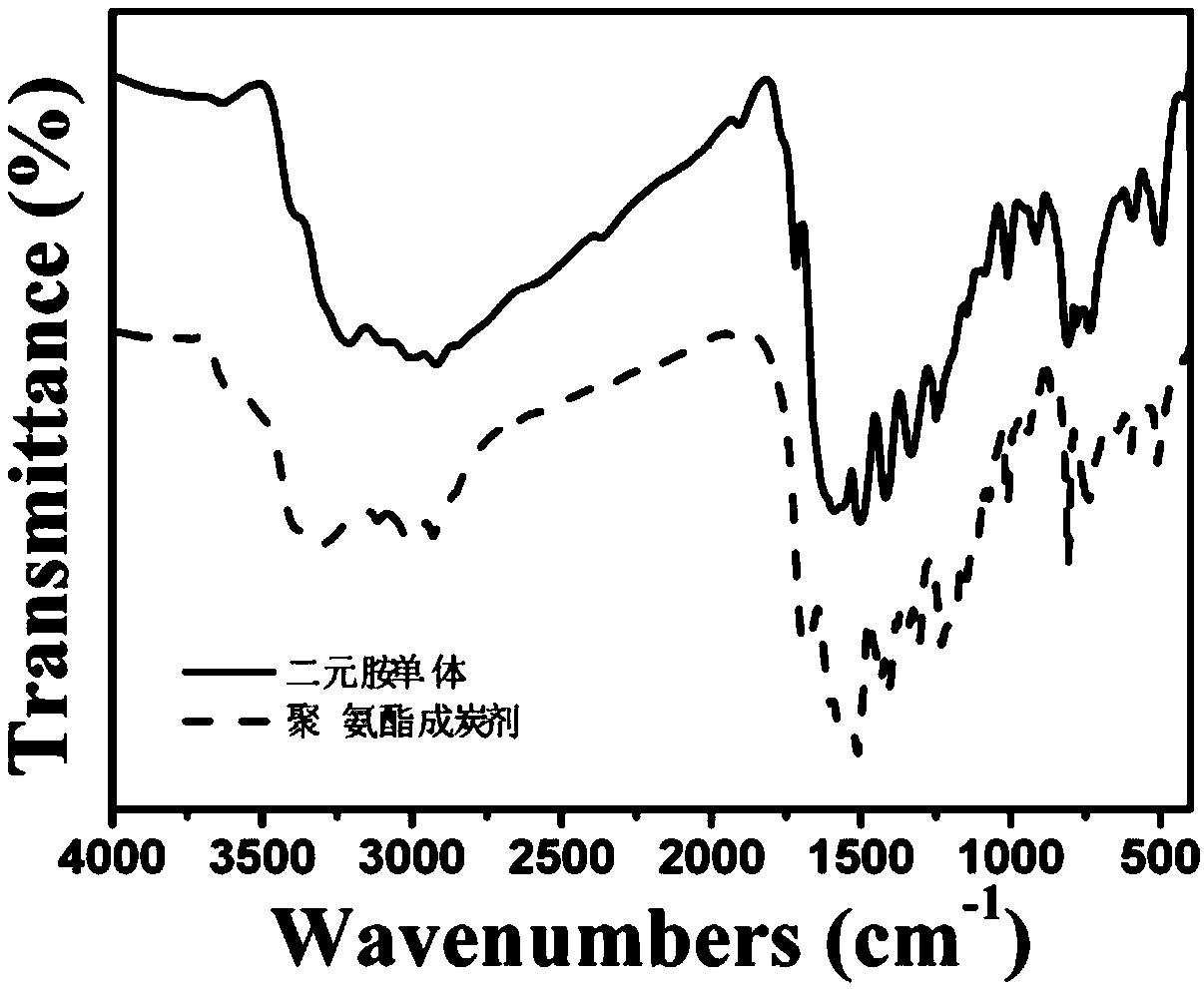
Triazine polyurethane carbon-forming agent containing furan ring side group and synthesis method thereof
A synthesis method and technology of triazines, which are applied in the field of triazine polyurethane char-forming agents and their synthesis, to achieve the effects of reducing synthesis costs, high yields, and improving biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
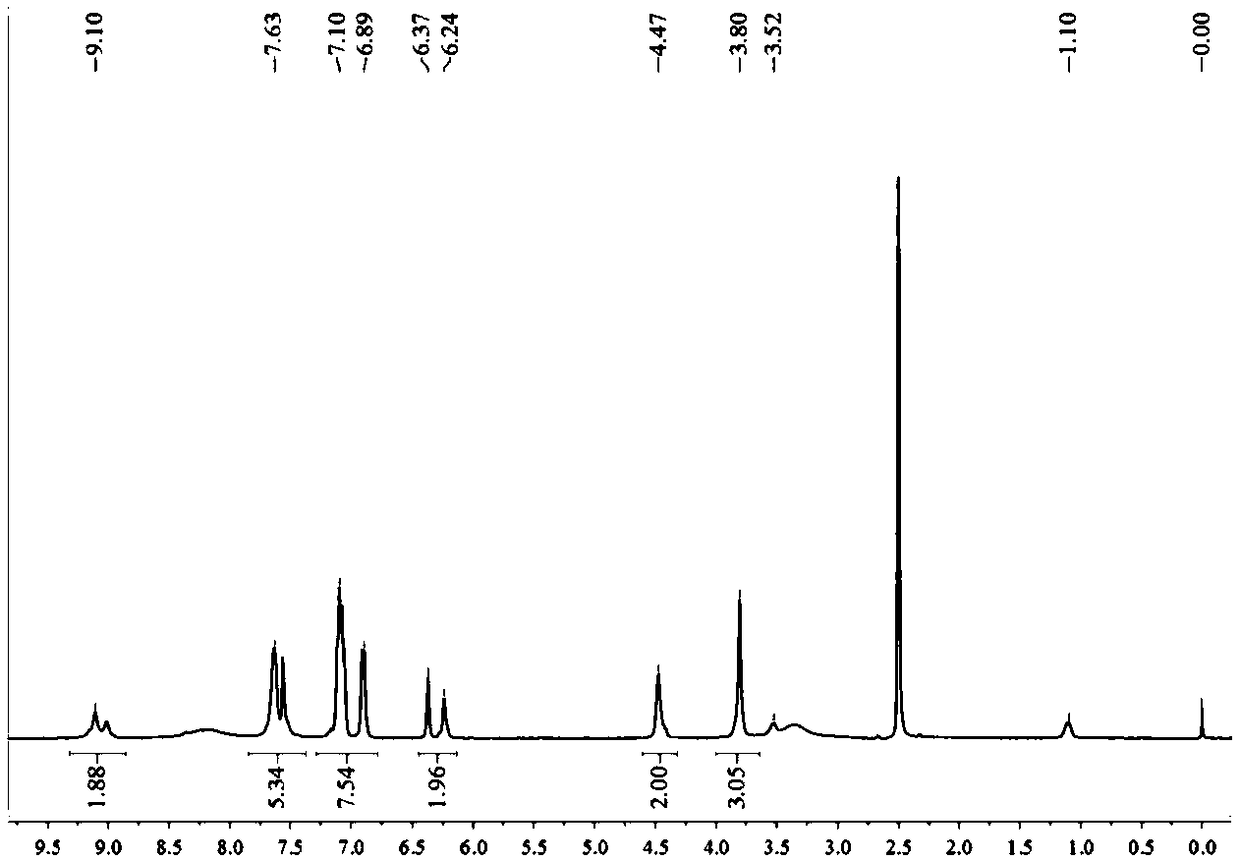
[0042] In a 1000mL three-necked flask, completely dissolve 1mol cyanuric chloride in 300mL1,4-dioxane, under ice-salt bath and nitrogen protection, dissolve 100mL1 of 1mol furfurylamine and 1mol triethylamine, The 4-dioxane solution was slowly dropped into it, and the dropping time was controlled to be 2 hours. After the dropping was completed, the reaction was continued for 2 hours in an ice-salt bath.
[0043] At room temperature, 100 mL of 1,4-dioxane solution dissolved with 2 mol of ethylenediamine and 2 mol of triethylamine was slowly dropped into it, and after the addition, the temperature was raised to 110° C. to continue the reaction for 8 h. After the reaction was completed, suction filtration was performed, and the filtrate was rotary evaporated to obtain a solid powder, which was washed with deionized water for 5 times, and product 1 was obtained after vacuum drying, which was set aside.
[0044] Dissolve 1 mol of product 1 completely dehydrated and 1 mol of 4,4-dip...
Embodiment 2
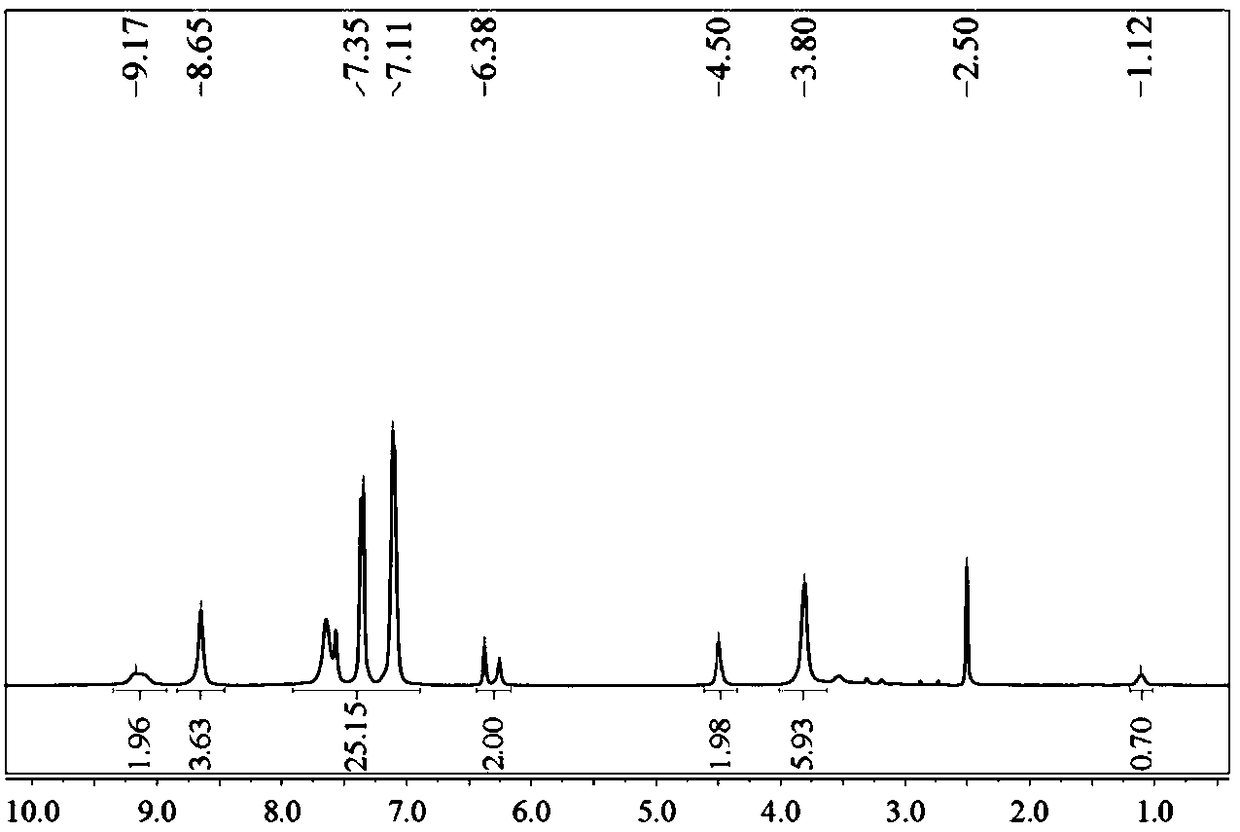
[0047] In a 1000mL three-necked flask, completely dissolve 1mol of cyanuric chloride in 400mL of acetonitrile, and slowly drop 100mL of acetonitrile solution dissolved in 1mol of methylfurylamine and 1mol of pyridine into it under ice-salt bath and nitrogen protection. Adding time is 2h. After the dropwise addition, continue to react in ice-salt bath for 2h.
[0048] At room temperature, 100 mL of acetonitrile solution dissolved with 2 mol of phenolphthalein and 2.6 mol of pyridine was slowly dropped into it, and after the addition was completed, the temperature was raised to 110° C. to continue the reaction for 8 h. After the reaction, filter with suction, spin evaporate the filtrate to obtain a solid powder, wash with deionized water for 5 times, and dry in vacuum to obtain the product 2, which is set aside.
[0049] Dissolve 1 mol of product 2 completely dehydrated and 1 mol of hexamethylene diisocyanate (HDI) in 400 mL of toluene dehydrated, mechanically stir evenly, add 2...
Embodiment 3
[0052] In a 1000mL three-necked flask, completely dissolve 1mol of cyanuric chloride in 300mL of 1,4-dioxane; The oxane solution was slowly dropped into it, and the dropping time was controlled to be 2h. After the dropping was completed, the reaction was continued for 2h in an ice-salt bath.
[0053] At room temperature, 100 mL of 1,4-dioxane solution dissolved with 2 mol of 4,4-diaminodiphenylmethane (DDM) and 2 mol of pyridine was slowly dropped into it. After the addition was completed, the temperature was raised to 110° C. to continue the reaction for 8 h. After the reaction, suction filtration was performed, and the filtrate was rotary evaporated to obtain a solid powder, which was washed with deionized water for 5 times, and the product 3 was obtained after vacuum drying, which was set aside.
[0054] Dissolve 1 mol of product 3 completely dehydrated and 1 mol of isophorone diisocyanate (IPDI) in 500 mL of N,N-dimethylformamide dehydrated, mechanically stir evenly, add 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com