Organic-inorganic composite all-solid electrolyte, a preparation method and application thereof
An inorganic composite, all-solid-state technology, used in solid electrolytes, electrolyte battery manufacturing, electrolytes, etc., can solve the problems that lithium batteries are difficult to process into thin, unsuitable batteries, poor mechanical properties, etc., to improve interface stability and long cycle. performance, good coulombic efficiency and capacity retention, and the effect of inhibiting the growth of lithium dendrites in the negative electrode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
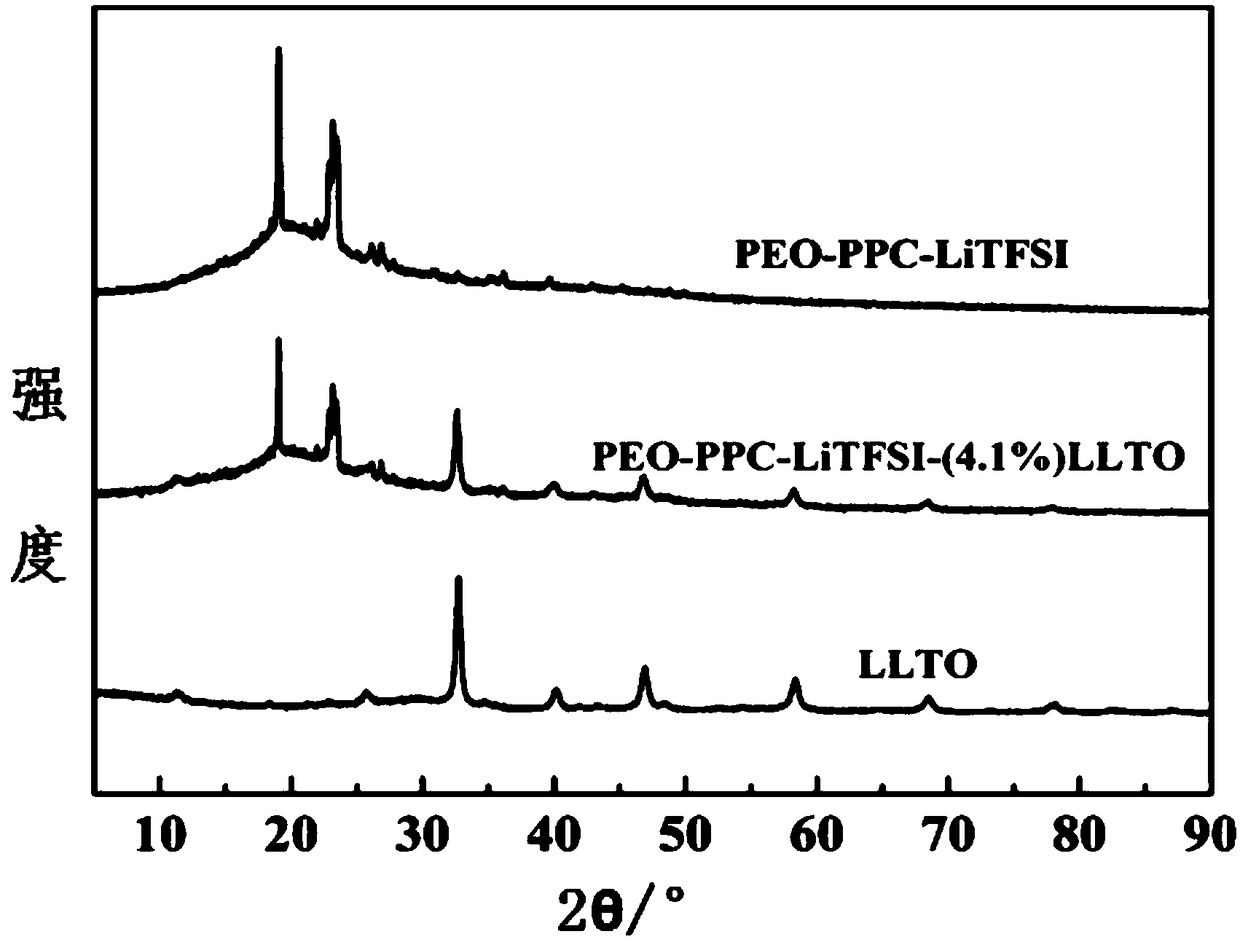
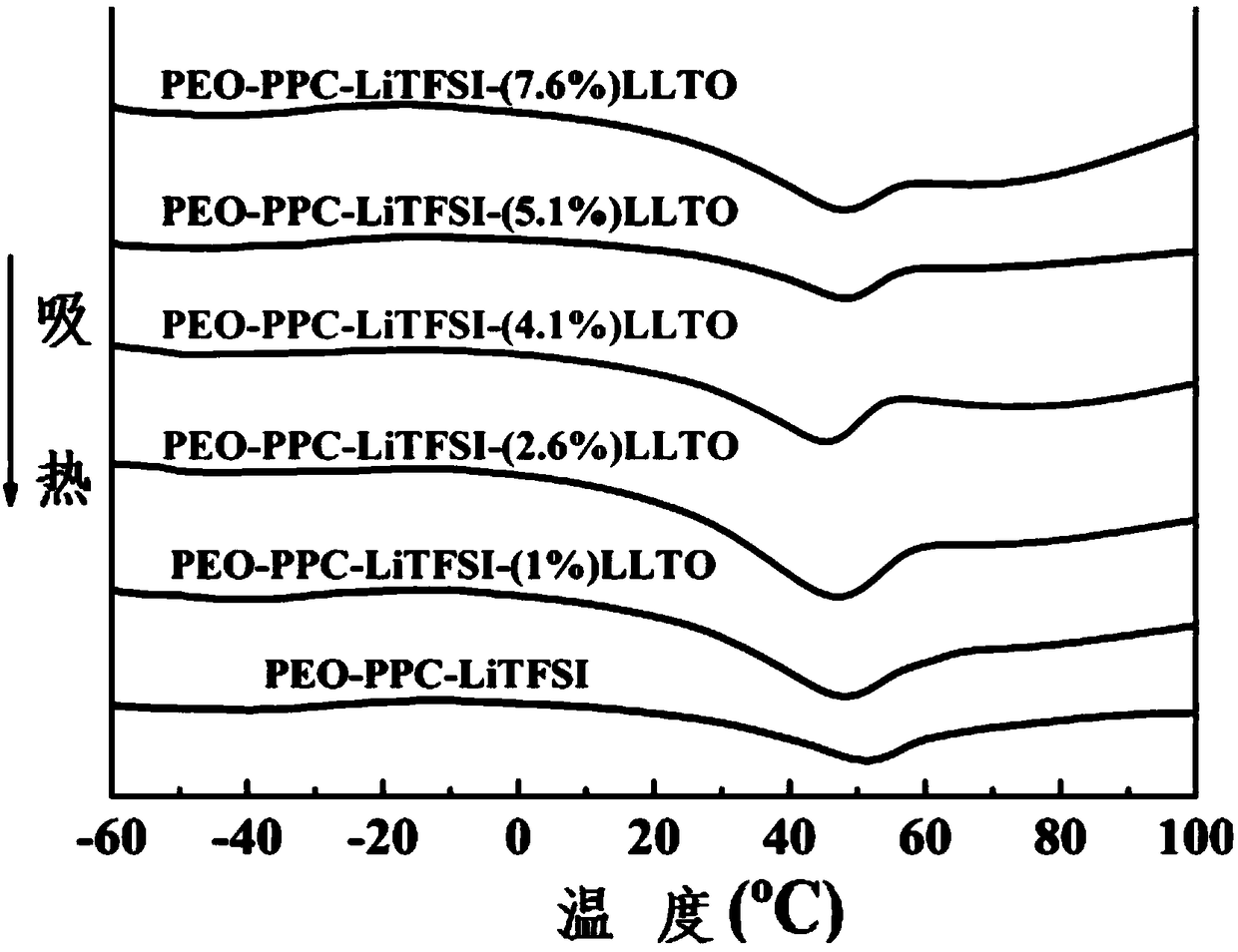
[0057] An organic-inorganic composite all-solid electrolyte (PEO-PPC-LiTFSI-(1%) LLTO) of the present invention is mainly composed of polyethylene oxide, polypropylene carbonate, and lithium trifluoromethanesulfonylimide (LiTFSI) and quasi-one-dimensional inorganic fast ion conductors (lithium lanthanum titanyl Li 0.33 La 0.557 TiO 3 Nanowire) composition, the mass percent composition of each component is: polyethylene oxide 53.2wt%, polypropylene carbonate 26.6wt%, quasi-one-dimensional lithium lanthanum titanyl Li 0.33 La 0.557 TiO 3 (LLTO) 1 wt%, lithium bistrifluoromethanesulfonylimide (LiTFSI) 19.2 wt%.
[0058] The preparation method of the organic-inorganic composite all-solid-state electrolyte of this embodiment includes the following steps:
[0059] (1) Weigh 0.36gLiTFSI and dissolve it in 50ml acetonitrile solvent to obtain 7.2g / L lithium salt solution;
[0060] (2) According to the amount of adding 0.4g quasi-one-dimensional inorganic fast ion conductor per li...
Embodiment 2
[0066] An organic-inorganic composite all-solid electrolyte (PEO-PPC-LiTFSI-(2.6%) LLTO) of the present invention is mainly composed of polyethylene oxide, polypropylene carbonate, and lithium trifluoromethanesulfonylimide (LiTFSI) and quasi-one-dimensional inorganic fast ion conductors (lithium lanthanum titanyl Li 0.33 La 0.557 TiO 3 Nanowire) composition, the mass percent composition of each component is: polyethylene oxide 52.4wt%, polypropylene carbonate 26.2wt%, quasi-one-dimensional lithium lanthanum titanyl Li 0.33 La 0.557 TiO 3 (LLTO) 2.6 wt%, lithium bistrifluoromethanesulfonimide (LiTFSI) 18.8 wt%.
[0067] The preparation method of the organic-inorganic composite all-solid-state electrolyte of this embodiment includes the following steps:
[0068] (1) Weigh 0.36gLiTFSI and dissolve it in 40ml of acetonitrile solvent to obtain a 9.0g / L lithium salt solution;
[0069] (2) According to the amount of adding 1.25g quasi-one-dimensional inorganic fast ion conducto...
Embodiment 3
[0075] An organic-inorganic composite all-solid electrolyte (PEO-PPC-LiTFSI-(4.1%) LLTO) of the present invention is mainly composed of polyethylene oxide, polypropylene carbonate, and lithium trifluoromethanesulfonylimide (LiTFSI) and quasi-one-dimensional inorganic fast ion conductors (lithium lanthanum titanyl Li 0.33 La 0.557 TiO 3 Nanowire) composition, the mass percent composition of each component is: polyethylene oxide 51.6wt%, polypropylene carbonate 25.8wt%, quasi-one-dimensional lithium lanthanum titanyl Li 0.33 La 0.557 TiO 3 (LLTO) 4.1 wt%, lithium bistrifluoromethanesulfonimide (LiTFSI) 18.5 wt%.
[0076] The preparation method of the organic-inorganic composite all-solid-state electrolyte of this embodiment includes the following steps:
[0077] (1) Weigh 0.36gLiTFSI and dissolve it in 50ml acetonitrile solvent to obtain 7.2g / L lithium salt solution;
[0078] (2) According to the amount of adding 1.6g quasi-one-dimensional inorganic fast ion conductor per ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com