Deuterated compound synthesis method
A deuterated compound and synthetic method technology, applied in the field of isotope labeling chemical synthesis, can solve the problems of harsh reaction conditions, flammable and explosive, expensive deuterium sources, etc., and achieve good substrate applicability, easy availability, and high-efficiency multi-deuterium effect of modernization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
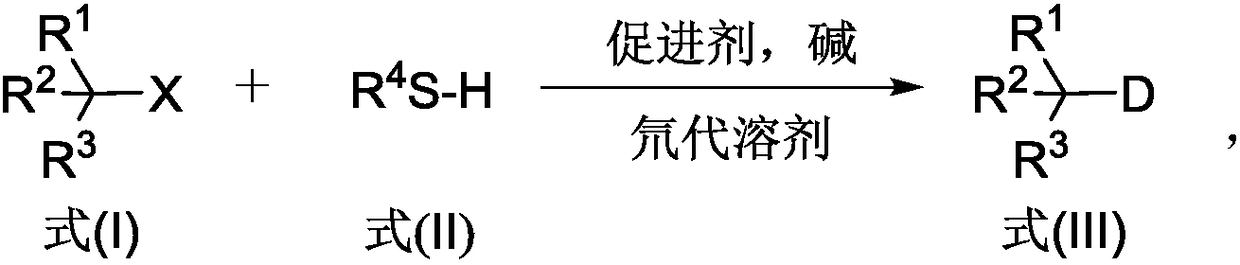
[0034] In general, the synthetic method of the deuterated compound provided by the invention specifically comprises the following steps:
[0035] (1) Halogenated compound shown in formula (I), sulfide shown in formula (II), promotor, alkali and deuterated solvent are mixed, and the amount of the substance of described sulfide (formula (II)) is described Halogenated compound (formula (I)) more than twice (that is, the ratio of the amount of sulfide and halogenated compound is greater than or equal to 2:1), the amount of the substance of the base is the amount of the halogenated compound (Formula (I)) is more than double (ie, the ratio of the amount of the base to the halogenated compound is greater than or equal to 1:1), and a mixed system of reaction raw materials is obtained.
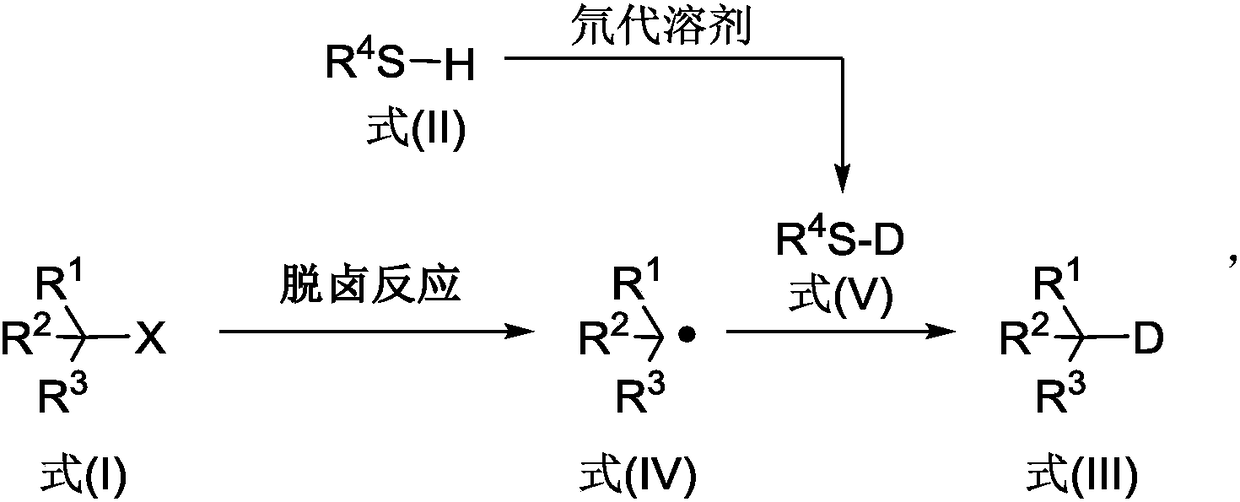
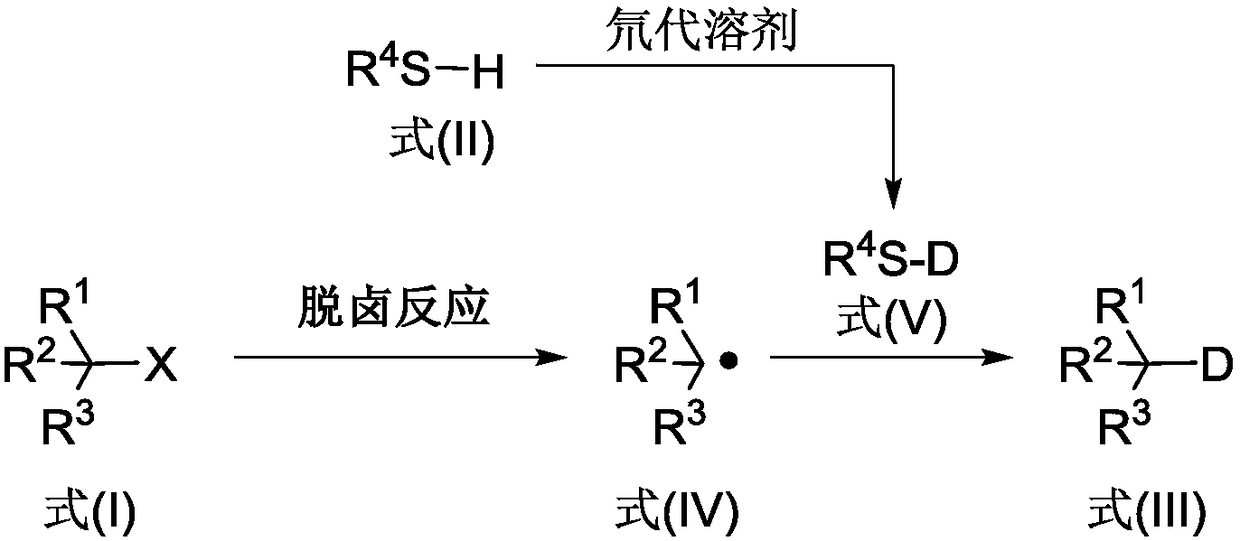
[0036] The reaction process of the above-mentioned method is as follows, wherein, the halogenated compound shown in formula (I) produces the free radical shown in formula (IV) under dehalogenation reac...
Embodiment 1
[0049] Embodiment 1: the synthesis of compound 1a
[0050]
[0051] a), weigh iodo compound 1 (30mg), bismuth trioxide (0.4mg, 1mol%), p-toluenethiol (p-toluenethiol, 55.2mg, 5equiv) in a 10mL reaction flask, and then Add acetonitrile (1.5ml), heavy water (1.5ml) and 2,6-lutidine (51.3μL, 5equiv), react under 1W blue LED light irradiation, and stop the reaction after iodo compound 1 is consumed (16h ). Extracted three times with ethyl acetate, combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain the crude product, separated and purified by column chromatography to obtain compound 1a (18.1 mg), the yield was 96%, and the deuterated rate was 97%.
[0052] b), iodo compound 1 (20mg), Pepto Bismol (containing C 7 h 5 BiO 4 ) (4.3mg, 5mol%) and p-methylthiophenol (36.8mg, 5equiv) were weighed in a 10mL reaction flask, and then acetonitrile (1.0ml), heavy water (1.0ml) and 2,6-dimethyl Basepyridine (34.2 μL, 5...
Embodiment 2
[0058] Embodiment 2: the synthesis of compound 2a
[0059]
[0060] a), weigh iodo compound 2 (30mg), bismuth trioxide (0.4mg, 1mol%), and p-methylthiophenol (53.8mg, 5equiv) in a 10mL reaction flask, and then add acetonitrile (1.5 ml), heavy water (1.5ml) and N,N-diisopropylethylamine (75.5μL, 5equiv) were reacted under 1W blue LED light irradiation, and the reaction was terminated after iodo compound 2 was consumed (9h). Extracted three times with ethyl acetate, combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain a crude product, separated and purified by column chromatography to obtain compound 2a (18.0 mg), the yield was 94%, and the deuterated rate was 97%.
[0061] b), weigh iodo compound 2 (30mg), bismuth trioxide (0.4mg, 1mol%), and p-methylthiophenol (53.8mg, 5equiv) in a 10mL reaction flask, and then add acetonitrile (1.5 ml), heavy water (1.5ml) and 2,6-lutidine (50.0μL, 5equiv), react under 1W b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com